D E A F G Oxysarcodexia Townsend, 1917 (D...
Transcript of D E A F G Oxysarcodexia Townsend, 1917 (D...

CARINA MARA DE SOUZA
DIVERSIDADE DE ESPÉCIES E ABORDAGEM FILOGENÉTICA DO GÊNERO
Oxysarcodexia Townsend, 1917 (DIPTERA: SARCOPHAGIDAE)
CAMPINAS
2014

ii

iii
UNIVERSIDADE ESTADUAL DE CAMPINAS
INSTITUTO DE BIOLOGIA
CARINA MARA DE SOUZA
DIVERSIDADE DE ESPÉCIES E ABORDAGEM FILOGENÉTICA DO GÊNERO
Oxysarcodexia Townsend, 1917 (DIPTERA: SARCOPHAGIDAE)
Orientadora: Profa. Dra. Patrícia Jacqueline Thyssen
CAMPINAS
2014

iv

v

vi

vii
Aos meus pais, razão de tudo.
"O que sabemos é uma gota, o que ignoramos é um oceano"
Isaac Newton

viii

ix
AGRADECIMENTOS
À minha família (especialmente papai Ariozano (in memorian), mamãe Maria José, e irmãos
Cassiano e Carolina) por compartilharem comigo esta etapa, por todo o apoio moral, amoroso e financeiro
durante este período e em todas as minhas decisões. Meu pai, apesar de não estar mais presente neste final,
foi de essencial à execução deste trabalho, além de um “ajudante” muito especial durante parte das coletas.
Sem o apoio e incentivo da minha mãe eu não teria o alicerce e força necessários para concluir essa
caminhada. Obrigada por serem meus exemplos, acreditarem em mim, pela compreensão nos momentos
de ausência (que foram muitos...), pelos conselhos e por todas as palavras motivadoras que me consolaram
e deram força nos momentos mais difíceis.
À inestimável Dra. Patricia J. Thyssen, grande amiga e mestre, pela orientação, oportunidade de
crescimento profissional e pessoal, apoio incondicional em todas as empreitadas dessa jornada, um “anjo-
da-guarda” na minha vida, tanto acadêmica quanto pessoal.
Ao Dr. Arício X. Linhares pelo apoio na execução desse projeto, pela convivência e singular
oportunidade de aprendizado, além das contribuições durante a qualificação, exame prévio e defesa.
Ao Dr. Thomas Pape pela oportunidade de estágio em seu laboratório e aprendizado no mundo
dos sarcofagídeos.
À Dra. Roseli Tuan pelos ensinamentos na área de biologia molecular.
À Dra. Cátia A. Mello-Patiu pela imensurável contribuição durante o estudo das Oxysarcodexia, a
afetuosa receptividade nas várias trocas de e-mails, em minha visita ao Museu Nacional e nos encontros
acadêmicos, além das relevantes contribuições na avaliação da tese.
Aos Drs. Marlene T. Ueta, Silmara Allegretti, Júlio Mendes, Carolina Reigada, Cláudio J. Von
Zuben, Paulo R. Bunde, Sérgio F. dos Reis e José Roberto Pujol-Luz pela disponibilidade em compor as
bancas dos exames de qualificação, prévio e defesa e por suas contribuições nestas etapas.
Aos amigos do Laboratório de Entomologia L2B, André Savino, Carolina Palanch, Cauê Mira,
Daniel Brancoli (Carioca), Danilo Ferraz, Fábio Rezende (Frango), Jandui Amorim, Maicon Grella
(Maicola), Marina Klemm (Nina), Marcela Alonso (Qualy), Mariana Nassu (Dori), Maria Lígia Paseto
(Armaria), Rafael Cedro, Thamiris Smania pelo ótimo ambiente de trabalho, pelas discussões científicas,
momentos de descontração e imensurável ajuda em coletas, criações de moscas, edição de imagens, dicas
na redação, dentre outras atividades, sem os quais parte do trabalho não seria possível. Prezadas amizades.
Aos queridos amigos feitos em Copenhagen, Eliana Buenaventura, Dave Cheung, Daniel
Whitmore, Ken Puliafico e Nesrine Akkari pelas aventuras no exterior e o apoio numa fase difícil.
Aos estimados colegas Karine Vairo, pelo material de Manaus; Matheus Camargo, pelo auxílio na
edição das imagens; Taís Madeira e Thamiris Barbosa, pela colaboração nos experimentos moleculares.
Aos prezados amigos da Pós-Graduação em Parasitologia e do departamento, em especial Luciana
Franceschi, Aline Rimoldi, Laura Gisloti, Rodrigo Labello e Raquel Palasio.
Aos amigos distantes, porém sempre presentes, que conviveram com minha ausência em vários
momentos e nunca se afastaram ou deixaram de me apoiar.
Aos demais professores e técnicos do departamento, em especial Tacilda S. Nalon (Tata), João
Batista e Letícia Duart, pelas contribuições durante as disciplinas e na realização deste trabalho.
À Universidade Estadual de Campinas por prover condições para minha formação acadêmico-
profissional e pelos subsídios fornecidos a esta pesquisa.
À CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) pela concessão da
bolsa de estudos (doutorado regular e programa sanduíche no exterior processo #0906/12-3).

x

xi
RESUMO
Um conspecto taxonômico com base nos machos de 85 espécies válidas pertencentes ao
gênero Oxysarcodexia Townsend, 1919 (Diptera: Sarcophagidae) é apresentado. Uma diagnose
do gênero e de cada espécie constituinte, distribuição geográfica, dados biológicos – quando
disponíveis –, e um apêndice pictórico são também inclusos nesse conspecto. Além do exame de
espécimes de Oxysarcodexia, uma ampla e minuciosa busca na literatura foi realizada para
abranger toda a informação disponível para esse gênero. Além disso, foram descritas seis
espécies novas de distribuição neotropical. Foi também realizada uma análise filogenética
baseada em caracteres morfológicos provenientes do exame de machos de 54 espécies
pertencentes ao gênero Oxysarcodexia a fim de investigar a assimetria fálica vista em algumas
espécies e a ocorrência de possíveis agrupamentos interespecíficos dentro do gênero. A
assimetria também foi estudada por meio de espécies modelo, com o auxílio da técnica de
microscopia eletrônica de varredura rotacional. A assimetria detectada foi do tipo direcional
sinistral, presente em oito espécies, restringindo-se aos lobos terminais da vésica e sendo
considerada uma homoplasia. Quanto aos agrupamentos de espécies, a topologia encontrada nas
árvores mais parcimoniosas obtidas mostrou certa similaridade com o proposto na literatura,
embora sem completa correspondência. Espera-se, com esse estudo, auxiliar no processo de
identificação dessas espécies, assim como contribuir para o melhor conhecimento desse gênero
de moscas.
Palavras-chave: Sarcofagídeo; Díptero – Morfologia; Inseto – Identificação; Microscopia
eletrônica de varredura.

xii

xiii
ABSTRACT
A taxonomic conspectus based on male specimens of 85 valid species of the genus
Oxysarcodexia Townsend, 1919 (Diptera: Sarcophagidae) is presented. A diagnosis for the genus
and for each species, the geographical distribution, the biological information – when available –,
and a pictorial appendix are included in this conspectus. Besides the species examination, a
thorough and wide search on the literature was performed in order to gather all information
available for this genus. Additionally, six new species of Neotropical distribution were described.
It was also performed a phylogenetic analysis based on males morphological characters of 54
species belonging to Oxysarcodexia genus, in order to study the phallic asymmetry present on
some species and the occurrence of possible interspecific grouping within the genus. The
asymmetry was also investigated through model species, with the help of rotational scanning
electron microscopy technique. The asymmetry detected was of sinistral directed type, present in
eight species, restricted to the terminal lobes of the vesica, and considered a homoplasy. About
the species grouping, the topology found on the most parsimonious trees obtained showed certain
similarity with the propositions of the literature, although without complete correspondence. We
expected with this study contribute with the species identification process and with the best
knowledge of this flesh fly genus.
Key-words: Sarcophagidae; Diptera – Morphology; Insects – Identification; Scanning electron
microscopy.

xiv
SUMÁRIO
1. INTRODUÇÃO ........................................................................................................................... 1
2. REVISÃO BIBLIOGRÁFICA ....................................................................................................... 3
3. OBJETIVOS .............................................................................................................................. 9
4. METODOLOGIA ...................................................................................................................... 11
5. CAPÍTULO I ........................................................................................................................... 13
Abstract ...................................................................................................................................... 13
Resumo ...................................................................................................................................... 14
Introduction ............................................................................................................................... 15
Material and Methods ................................................................................................................ 16
Generic Definition ..................................................................................................................... 18
Remarks ..................................................................................................................................... 19
Genus Diagnosis ........................................................................................................................ 20
Species awaiting status confirmation ........................................................................................ 23
Taxonomy .................................................................................................................................. 23
Final considerations ................................................................................................................. 132
References ............................................................................................................................... 132
Appendices .............................................................................................................................. 147
6. CAPÍTULO II ........................................................................................................................ 179
RESUMO ................................................................................................................................... 179
INTRODUÇÃO ............................................................................................................................ 180
MATERIAL E MÉTODOS ............................................................................................................. 182
RESULTADOS ............................................................................................................................ 183
DISCUSSÃO ............................................................................................................................... 185
REFERÊNCIAS BIBLIOGRÁFICAS ................................................................................................ 190
APÊNDICE ................................................................................................................................. 204
7. CONSIDERAÇÕES FINAIS ..................................................................................................... 217
8. REFERÊNCIAS BIBLIOGRÁFICAS ......................................................................................... 219

1
1. INTRODUÇÃO
O gênero Oxysarcodexia Townsend, 1917 (Diptera: Sarcophagidae) está distribuído
majoritariamente na região Neotropical, sendo frequentemente relatada a associação destas
moscas a substratos em decomposição, como fezes e carcaças, despertando grande interesse
em diversas áreas como a forense, médica e veterinária. Apesar disso, não é incomum a
identificação das espécies ficar restrita ao nível genérico ou ser errônea, devido à existência de
espécies com caracteres morfológicos muito similares e facilmente confundíveis quando
inexiste familiaridade com as diferentes espécies.
Nesse contexto foi elaborado o primeiro capítulo desta tese, apresentando um conspecto
taxonômico para machos de Oxysarcodexia. Os dados foram formatados de modo a
possibilitar o fácil reconhecimento das diferentes espécies a partir do oferecimento de uma
diagnose do gênero, listagem das espécies válidas, sinonimização de nomes, origem de
materiais tipo e seus depositários, diagnose de cada espécie, discussões sobre caracteres
morfológicos similares para diferentes espécies, distribuição geográfica, dados biológicos e
um banco de dados pictórico a fim de facilitar o reconhecimento das diferentes espécies. Este
capítulo contemplou também a descrição de seis espécies novas a partir do exame de material
depositado em museus (Oxysarcodexia n. sp. 1, coletada na Colômbia e Equador;
Oxysarcodexia n. sp. 2, coletada na Guiana Francesa; Oxysarcodexia n. sp. 3, coletada na
Costa Rica; Oxysarcodexia n. sp. 4, coletada no Brasil; Oxysarcodexia n. sp. 5, e
Oxysarcodexia n. sp. 6, ambas coletadas no Equador).
A partir da análise morfológica dos machos, especialmente da terminália, observou-se a
ocorrência de assimetria na genitália. Por ser uma condição não usualmente observada para os
insetos, que, por via de regra, apresentam simetria bilateral, a investigação desta condição
motivou a estruturação do segundo capítulo. Foi proposta uma análise filogenética baseada em
uma matriz de caracteres morfológicos compilados a partir do estudo de machos de diferentes
espécies, a fim de rastrear a ocorrência da assimetria no gênero Oxysarcodexia. Além disso,
micrografias rotacionais foram produzidas a partir de imagens de microscopia eletrônica de
varredura para Oxysarcodexia fringidea (Curran & Walley, 1934), Oxysarcodexia timida
(Aldrich, 1916) e Oxysarcodexia varia (Walker, 1836), utilizadas como modelos para a
análise das alterações morfológicas consequentes da assimetria.

2
Espera-se que a investigação da diversidade e a abordagem filogenética para as espécies
de Oxysarcodexia contribuam para o melhor entendimento deste grupo e de suas relações
interespecíficas, representando uma motivação para a realização de outros estudos.

3
2. REVISÃO BIBLIOGRÁFICA
A ordem Diptera (Insecta: Arthropoda) compreende cerca de 160.590 espécies,
incluindo aproximadamente 3.800 espécies fósseis, consideradas subestimadas (Zhang, 2013).
Na região Neotropical há a ocorrência de mais de 31.000 espécies (Evenhuis et al., 2007 apud
Amorim, 2009). Estima-se, no entanto, que esse número seja bem maior (Brown et al., 2009),
até cinco vezes mais para algumas famílias (Amorim, 2009), devido a áreas ainda não
amostradas, espécies coletadas que ainda aguardam descrição e ao número restrito de
taxonomistas estudando determinadas espécies (Rafael et al., 2009).
A família Sarcophagidae (Diptera: Muscomorpha) possui aproximadamente 355 gêneros
e em torno de 3.100 espécies descritas mundialmente, sendo que cerca de 870 espécies
ocorrem na região Neotropical e 270 apenas no Brasil (Evenhuis et al. 2007 apud Pape et al.
2009; Carvalho et al. 2012). É reconhecidamente dividida em três subfamílias,
Miltogramminae, Paramacronychiinae e Sarcophaginae, com uma possível relação de grupo-
irmãos entre as duas últimas e monofiletismo reconhecido filogeneticamente para
Sarcophaginae (Pape, 1996).
A subfamília Sarcophaginae engloba moscas com tamanho variável, embora a coloração
externa seja razoavelmente homogênea, consistindo em um tórax acinzentado com três faixas
pretas e abdômem com padrão enxadrezado. A distribuição e diversidade de espécies de
sarcofagídeos são, de maneira geral, diretamente proporcionais a regiões de clima quente,
sendo, consequentemente, bastante reduzidas em regiões subárticas, assim como em ambientes
insulares e, como esperado, inexistentes nas regiões árticas (Shewell, 1987; Pape, 1996). Os
hábitos das espécies inclusas nessa subfamília são amplamente diversificados, variando entre a
coprofagia, a saprofagia, a predação e o parasitismo (Shewell, 1987; Pape, 1996; Pape &
Dahlem, 2010; Carvalho et al., 2012).
Roback (1954), ao estudar a evolução e taxonomia de Sarcophaginae com base na
similaridade morfológica de caracteres externos do corpo, especialmente os da terminália dos
machos, propôs a divisão desta subfamília em duas tribos, Agriini e Sarcophagini, e diversas
subtribos. Partindo deste estudo, Lopes (1969) propôs uma nova divisão que considera a
nomeação de seis tribos: Microcerellini, Notochaetini, Raviniini, Sarcophagini, Sarcophagulini
e Tephromyiini. Ao considerar o desenvolvimento da mandíbula e do arco clipeal das larvas
de primeiro estádio, associado à análise morfológica de machos e fêmeas, especialmente de

4
caracteres da genitália, Lopes (1982) acrescentou as tribos Cuculomyiini, Impariini e
Sarcodexiini (por revisão de status) e Sarothromyiopsini (por nova proposição), resultando em
11 tribos inclusas na subfamília Sarcophaginae. Essa divisão foi corroborada por Verves
(1989), que revisou as subtribos e apresentou uma chave para a identificação das tribos
Cuculomyiini, Emblemasomatini, Impariini, Johnsoniini, Microcerellini, Protodexiini,
Raviniini, Sarcodexiini, Sarcophagini, Sarothromyiini e Sarothromyiopsini.
O gênero Oxysarcodexia Townsend, 1917 é um dos gêneros de Sarcophaginae que
apresenta maior riqueza de espécies na região Neotropical (Lopes, 1969; Verves, 1989; Pape,
1996). Juntamente com Nephochaetopteryx Townsend, 1934, Orosarcophaga Townsend,
1927 [naquele momento ainda considerado um gênero e, posteriormente, reclassificado como
subgênero de Lepdidodexia Brauer & Bergenstamm, 1891 (Pape, 1996)], Oxyvinia Dodge,
1966 e Ravinia Robineau-Desvoidy, 1863, acomodado na tribo Raviniini.
A presença de uma projeção triangular (em forma de “dente”) do tubo fálico, logo acima
da vésica, é considerada a única apomorfia que caracteriza o monofiletismo de Oxysarcodexia
(Giroux et al., 2010). As espécies inclusas nesse gênero são consideradas, de maneira geral,
coprófagas, mas podem também ser encontradas associadas a carcaças (Pape & Dahlem 2010;
Carvalho et al. 2012).
Os dípteros muscóideos, de maneira geral, inclusos os sarcofagídeos, têm acompanhado
a população humana ao longo da história, estabelecendo durante muito tempo associações
harmônicas e desarmônicas. Essas relações eventualmente repercutem em problemas tanto
para a área da saúde quanto para a econômica, por exemplo, quando ocasionam e/ou veiculam
doenças e óbito ao atingirem o homem ou seus animais domésticos (Greenberg, 1973).
As relações que os dípteros possuem com o ambiente humano podem ser classificadas
como sinantropia, um fenômeno ecológico diretamente ligado à biocenose humana, a qual é
influenciada pelas ações antrópicas sobre o ambiente e refletida pelas condições de
antropobiocenoses (habitações humanas e seus animais domésticos) e agrobiocenoses
(pastagens, clareiras florestais, monoculturas e policulturas) (Greenberg, 1971; Ferreira,
1978). Nessas condições, na procura por disponibilidade de recursos alimentares ou sítios para
deposição de seus ovos ou larvas, os dípteros podem encontrar-se associados ao lixo ou às
fezes, tornando-se potenciais carreadores e propagadores de patógenos como vírus, bactérias,
protozoários e helmintos (Greenberg, 1971; 1973; Thyssen et al., 2004).

5
Outras espécies acarretam danos diretos à saúde, quando são agentes causadores de
miíases, popularmente conhecidas como bicheiras (Linhares & Thyssen, 2007). Esta doença é
caracterizada pela infestação, em geral do tecido cutâneo e subcutâneo de vertebrados, por
estágios imaturos de moscas que se alimentam por um curto período de tecidos vivos ou
necrosados de seus hospedeiros (Zumpt, 1965). Apesar das varejeiras estarem mais
comumente envolvidas nesses quadros, dípteros sarcofagídeos também podem ocasionar
miíase humana. Um caso de miíase uretral causada por larvas de Peckia (Sarcodexia) lambens
(Wiedemann, 1830) foi relatado em Belém, Pará (Leão et al. 1996) (Diptera: Sarcophagidae).
Em São João de Meriti, Rio de Janeiro, um caso de infestação auricular, cujas envolvidas
pertenciam à espécie Oxysarcodexia amorosa (Schiner, 1868) (Diptera: Sarcophagidae), foi
reportado por Figueiredo e colaboradores (2002). Casos de miíases humanas (orotraqueal,
auricular e furuncular na região da axila, por exemplo) causados por larvas de Wohlfahrtia
magnifica (Diptera: Sarcophagidae) tem sido relatados com certa frequência em países
europeus (por exemplo, Çiftçioğlu, Altintaș & Haberal 1997; Tuygun et al. 2009; Yazgi et al.
2009). Além disso, as espécies de sarcofagídeos Sarcophaga (Bercaea) haemorrhoidalis,
Sarcophaga (Bercaea) cruentata (Meigen, 1826) e Peckia (Peckia) chrysostoma (Wiedemann,
1830) também já foram associadas a casos de míiases humanas (por exemplo, Guimarães &
Papavero 1999; Turhan et al. 2007; Dutto & Bertero 2010).
Os tecidos de animais mortos podem ser atrativos para uma grande variedade de insetos
sarcossaprófagos, sobretudo dípteros, pelo fato da matéria orgânica constituir uma fonte
alimentar com alto valor proteico para o desenvolvimento de imaturos e nutrição dos adultos
(Nuorteva, 1977; Smith, 1986). Desse modo, a partir dos trabalhos realizados na segunda
metade do século XIX por Mégnin (1894), levantando informações biológicas e ecológicas
para demonstrar o potencial sobre a contribuição dos insetos em investigações de caráter
médico-legal, foi criada a Entomologia Forense. Nessa vertente, os dípteros ganham maior
destaque por serem os primeiros a chegar a uma cena de crime, já que possuem órgãos
sensitivos altamente especializados para a detecção de odores e podem ovipor ou larvipor em,
aproximadamente, até 10 minutos após a morte (Campobasso et al., 2001).
Alguns dos inventários da dipterofauna de determinados ambientes e de trabalhos sobre
os dípteros encontrados visitando ou criando-se em carcaças e cadáveres em decomposição
resultaram em listagens nas quais a identificação taxonômica em nível de espécie dos

6
sarcofagídeos, incluindo aqueles pertencentes ao gênero Oxysarcodexia, não foi alcançada em
sua maioria. Nesses casos, os dados registrados foram referentes apenas à abundância e
frequência de “morfotipos” em ambientes urbanos e silvestres (Carrera 1944; Cornaby 1974;
Dias et al., 1984; Monteiro-Filho & Penereiro, 1987; Souza & Linhares, 1997; Carvalho et al.,
2000; Couri et al., 2000; Pamplona et al., 2000; Carvalho & Linhares, 2001; Carvalho et al.,
2004; Perez et al., 2005; Costamagna et al., 2007; Cruz 2008; Amat 2010; Battán-Horenstein
et al., 2010; Beuter et al., 2012; Sousa et al., 2011; Battán-Horenstein et al., 2012; Ramírez-
Mora et al., 2012).
O produto da morfotipagem, isto é, a criação de categorias abstratas como „morfotipos‟,
as quais não apresentam especificidade necessária para diferenciar um ser de outro, prejudica
o avanço de quaisquer outros trabalhos que investiguem a biologia, ecologia ou dinâmica dos
mais diversos organismos na natureza. Isto explicaria porque, com a grande diversidade de
espécies que há no Brasil, um conhecimento mais aprofundado relativo à bionomia,
identificação e classificação dos dípteros de importância médica, veterinária e forense ainda é
incipiente e a realização de tais estudos é de grande relevância para a formação de bancos de
dados.
Contudo, vale ressaltar que a identificação de diferentes espécies nem sempre é fácil e
corriqueira. A diversidade e as minúsculas diferenças morfológicas observadas entre as várias
espécies, além da ausência de chaves taxonômicas para certos grupos e a insuficiência na
descrição dos caracteres morfológicos em algumas já existentes, aliados ao pequeno número
de especialistas/taxonomistas existente para determinados grupos são alguns dos fatores que
contribuem para a complexidade do processo de identificação (Liu & Greenberg, 1989; Rafael
et al., 2009).
A sistemática filogenética surge, então, numa tentativa de dimensionar a diversidade por
meio da descrição da variedade e da busca por um padrão de ordem nos processos envolvidos
na diversificação dos organismos, visando à ordenação por meio de um sistema hierárquico
(Amorim, 2002). A região Neotropical, por exemplo, abrange uma complexidade florística e
faunística enorme, tornando a reconstrução dos padrões envolvidos no processo contínuo de
mudanças que geram a diversidade das espécies uma tarefa difícil (Amorim, 2012). Isso
mostra, em suma, a relevância do conhecimento mais detalhado das espécies no que diz
respeito aos seus caracteres morfológicos, biológicos ou ecológicos, assim como de variações

7
intraespecíficas e das relações interespecíficas com as demais espécies para várias áreas do
conhecimento, como a entomologia médica, veterinária e forense, a biogeografia, a evolução,
entre outras.

8

9
3. OBJETIVOS
1. Realizar um conspecto taxonômico sobre o gênero Oxysarcodexia Townsend, 1917
(Diptera: Sarcophagidae) incluindo informações inerentes a cada espécie, como nome válido e
sinonímias, localidade onde o material tipo foi coletado e instituição depositária, diagnose para
identificação dos machos, distribuição geográfica e informações biológicas, quando
disponíveis.
2. Produzir um banco de dados pictórico para machos de espécies de Oxysarcodexia.
3. Estudar a ocorrência de assimetria na genitália de alguns machos pertencentes a este
gênero.
4. Propor uma hipótese de relação filogenética para as espécies de Oxysarcodexia com
base na morfologia externa dos machos.

10

11
4. METODOLOGIA
Para a elaboração de um conspecto taxonômico e uma matriz de caracteres morfológicos
foram examinados espécimes machos pertencentes às espécies do gênero Oxysarcodexia.
Essas moscas foram provenientes de material de coleção científica depositado nas seguintes
instituições:
CE-TdeA – Centro Tecnológico de Antioquia, Instituição Universitária (Medellín,
Colômbia);
L2B-DBA – Coleção de referência do Laboratório de Entomologia do Departamento de
Biologia Animal, Universidade Estadual de Campinas (Campinas, Brasil)
MNRJ – Museu Nacional/Universidade Federal do Rio de Janeiro (Rio de Janeiro, Brasil);
NRM – Museu de História Natural Sueco (Estocolmo, Suécia);
ZMUC – Museu de Zoologia de Copenhagen (Copenhagen, Dinamarca).
O registro pictórico da terminália dessas espécies, em vista lateral, posterior e anterior
(este último, sempre que possível), assim como do hábito em vista lateral, foi realizado com o
auxílio de uma câmera digital Leica DELUX 3®
(10 megapixels) acoplada a um
estereomicroscópio Leica S8AP0®
e de uma câmera digital Carl Zeiss AXIOCAM MRc®
(5
megapixels) acoplada a um esteromicroscópio Carl Zeiss STEREO DISCOVERY.V12®
. Cada
fotografia foi produzida após a aquisição de imagens com foco estendido, agrupadas com o
auxílio do programa Zerene Stacker®
. Para as espécies sem material disponível para exame, a
melhor ilustração disponível na literatura foi apresentada, com edições sempre que necessário.
Além da informação pictórica acerca de cada espécie, uma minuciosa revisão da
literatura publicada sobre o gênero Oxysarcodexia foi realizada para a compilação de uma lista
taxonômica incluindo as espécies válidas, sinonímias, localidade tipo, depositários do material
tipo, diagnose, distribuição geográfica e notas sobre a biologia de cada espécie, sempre que
disponível. A descrição dos caracteres morfológicos externos seguiu terminologia proposta
por McAlpine (1981), ao passo que para a terminália masculina, seguiu-se as proposições de
Mello-Patiu & Pape (2000) e Giroux et al. (2010).
A matriz de caracteres para machos de Oxysarcodexia foi produzida com o auxílio do
programa Mesquite versão 2.75 (Maddison & Maddison, 2011), incluindo caracteres
morfológicos externos e da terminália, escolhidos após exame comparativo entre as espécies.

12
As análises filogenéticas foram realizadas com auxílio do programa TNT (Goloboff et al.,
2008), por meio de análises por busca tradicional e por novas tecnologias de busca. As árvores
mais parcimoniosas foram geradas considerando pesos iguais e pesos implicado para os
carcteres, incluindo os valores de suporte Bremer para os ramos, tanto para a análise da matriz
completa quanto da matriz considerando apenas caracteres da terminália.
Para analisar as alterações morfológicas consequentes da assimetria, as espécies
Oxysarcodexia fringidea (Curran & Walley, 1934), Oxysarcodexia timida (Aldrich, 1916) e
Oxysarcodexia varia (Walker, 1836), que apresentam vésicas assimétricas, foram submetidas
à microscopia eletrônica de varredura (MEV) para a produção de micrografias rotacionais. A
terminália dos espécimes foi cuidadosamente dissecada, clarificada em ácido lático 80% e
devidamente preparada para a produção de micrografias em microscópio eletrônico de
varredura do tipo “JEOL-JSM-6335F”, localizado no Museu de Zoologia da Universidade de
Copenhagen, Copenhagen, Dinamarca. A confecção de micrografias rotacionais interativas foi
realizada de acordo com a metodologia proposta por Cheung et al. (2013). As imagens foram
então agrupadas em uma animação e as micrografias rotacionais disponibilizadas na web, com
o auxílio de um plug-in específico.

13
5. CAPÍTULO I
ON Oxysarcodexia (DIPTERA: SARCOPHAGIDAE): A TAXONOMIC CONSPECTUS WITH THE
DESCRIPTION OF SIX NEW SPECIES
SOBRE O GÊNERO Oxysarcodexia (DIPTERA: SARCOPHAGIDAE): CONSPECTO TAXONÔMICO
COM A DESCRIÇÃO DE SEIS ESPÉCIES NOVAS
CARINA MARA DE SOUZA1, THOMAS PAPE
2 & PATRICIA JACQUELINE THYSSEN
3
1 Department of Animal Biology, University of Campinas - UNICAMP, POB 6109, PC 13083-
970, Campinas, São Paulo, Brazil. E-mail: [email protected]
2 Natural History Museum of Denmark, Universitetsparken 15, DK - 2100 Copenhagen,
Denmark. E-mail: [email protected]
3 Department of Microbiology and Biology, Federal University of Pelotas - UFPel, POB 354,
PC 96010-900, Pelotas, Rio Grande do Sul, Brazil. E-mail: [email protected]
Abstract
The genus Oxysarcodexia (Diptera: Sarcophagidae) is one of the most species-rich
genera of Neotropical flesh flies, with a few species occurring also in Nearctic, Australasian
and Oceanian Regions. Species within this genus are considered dung-breeding, although a
great part of biological information is still unknown. A taxonomical conspectus based on male
specimens of Oxysarcodexia genus is presented, including diagnosis of each species,
geographical distribution and biological data, when available, besides a pictorial appendix. It
is currently recognized a total of 89 valid species belonging to Oxysarcodexia genus, including
six new here described: Oxysarcodexia n. sp. 1 (Colombia and Ecuador), Oxysarcodexia n.
sp. 2 (French Guiana), Oxysarcodexia n. sp. 3 (Costa Rica), Oxysarcodexia n. sp. 4 (Brazil),
Oxysarcodexia n. sp. 5 (Ecuador) and Oxysarcodexia n. sp. 6 (Ecuador). On the moment,

14
Oxysarcodexia aureiceps (Macquart, 1855), Oxysarcodexia dorisae Dodge, 1965,
Oxysarcodexia flavifrons (Macquart, 1846) and Oxysarcodexia neivae Mattos, 1919 are
considered species awainting status confirmation once original descriptions are based only on
female specimens, some of type material are in very bad conditions, and until a wide and
thorough study of females is conducted.
Key words: Flesh flies, dung-breeding flies, Neotropical Region, new records.
Resumo
O gênero Oxysarcodexia (Diptera: Sarcophagidae) é um dos gêneros de sarcofagídeos
que apresenta grande riqueza de espécies que ocorrem majoritariamente na região Neotropical,
com poucas ocorrendo também nas regiões Neártica, Australásia e Oceânica. As espécies
inclusas nesse gênero são consideradas coprófagas, embora a biologia de muitas espécies
ainda permaneça desconhecida. Um conspecto taxonômico com base nos machos do gênero
Oxysarcodexia é apresentado, incluindo diagnose de cada espécie, distribuição geográfica e
dados biológicos, quando disponíveis, além de um apêndice pictórico. São reconhecidas 89
espécies válidas de Oxysarcodexia, incluindo seis espécies novas que se encontram aqui
descritas: Oxysarcodexia n. sp. 1 (Colômbia e Equador), Oxysarcodexia n. sp. 2 (Guiana
Francesa), Oxysarcodexia n. sp. 3 (Costa Rica), Oxysarcodexia n. sp. 4 (Brasil),
Oxysarcodexia n. sp. 5 (Equador) e Oxysarcodexia n. sp. 6 (Equador). Momentaneamente as
espécies Oxysarcodexia aureiceps (Macquart, 1855), Oxysarcodexia dorisae Dodge, 1965,
Oxysarcodexia flavifrons (Macquart, 1846) e Oxysarcodexia neivae Mattos, 1919 foram
consideradas espécies aguardando confirmação de status devido às descrições originais serem
baseadas exclusivamente em fêmeas, alguns dos materiais tipo estarem comprometidos por
problemas de conservação e até que um amplo e minucioso estudo das fêmeas desse gênero
seja realizado.
Palavras-chave: Sarcofagídeos, moscas coprófagas, região Neotropical, novos registros.

15
Introduction
Sarcophagidae (Diptera: Brachycera) is a family comprising three subfamilies,
Paramacronychiinae, Miltogramminae and Sarcophaginae, with about 3,100 described species.
Approximately 870 have been recorded for the Neotropical Region (Evenhuis et al. 2007 apud
Pape et al. 2009). However, richness of flesh flies might follow the same pattern of Diptera in
general, which is estimated be much higher than the number of described species. The low
number of taxonomists and areas still poorly or not sampled are limiting factors to the
knowledge of actual number of species (Brown 2009; Carvalho et al. 2012). The ecological
relationships of flesh flies vary from parasitism (such as myiasis and parasitoids), predatism
(on other insects, snails, earthworms or spider egg sacs) to feeding in decomposing organic
matter (as vertebrate/invertebrate carcasses and feces) (Pape & Dahlem 2010; Carvalho et al.
2012).
Oxysarcodexia, a genus belonging to the subfamily Sarcophaginae, is one of the most
species-rich genera of Neotropical Sarcophagidae, with about 80 species (Pape 1996; Soares
& Mello-Patiu 2010) and a few occur also in Nearctic, Australasian and Oceanian Regions
(Lopes 1973b, Lopes & Tibana 1987; Pape 1996). Species of this genus play an important
ecological role by being associated with decomposing organic material, as feces of mammals
or birds (dung-breeding) and carcasses (attracted fauna and carrion-breeding) (Pape & Dahlem
2010; Carvalho et al. 2012). Furthermore, it is one of the most frequently recorded and, in
many cases, also most diverse flesh fly genus in about 80 studies recorded in the literature,
dealing with forensic entomology, synanthropy or surveys of dipteran species, especially in
the Neotropical Region (e.g. Dodge & Seago 1954; Linhares 1981; D‟Almeida 1984; Dias et
al. 1984a; Oliveira-Costa et al. 2001; Barros et al. 2008; Rosa et al. 2011). Despite of this
wide occurrence, these flies remain identified in many papers only to genus level (e.g.
Cornaby 1974; Wolff et al. 2001; Pérez, Duque & Wolff 2005; Amat 2010; Horenstein et al.
2010), evidencing the issue of identifying species with a high level of homologies and
similarities. Establishment and maintenance of colonies of different Oxysarcodexia species at
laboratory is also a difficult task, mainly due to the difficulty of having flies to mate under
artificial conditions (Lopes 1973b).
Thus, the aim of this work was review the genus Oxysarcodexia, in order to provide
taxonomic, morphologic, pictorial and biologic information of the species included in this

16
taxon, with several species that have medical, veterinary and forensic importance. This
taxonomic conspectus is focused basically on male adults. Immatures are poorly known and
are scantily documented. Larval descriptions are found only for six species (see Knipling
1936; Lopes 1943; Wharton & Moon 1979; Lopes & Leite 1986; 1987; Leite & Lopes 1987),
although information on larval stages is reported by Lopes (1973b) for another 24 species
mention no details though. Females are known and have been described for some species (see
Walker 1857; Lopes 1933; 1938; 1939; 1946b; 1973a; 1973b; 1975a; 1975b; 1975c; 1975d;
1976; 1978; 1985; Blanchard 1939; 1942; Lopes & Albuquerque 1955; Dodge 1956; 1965;
1966; 1968; Tibana & Mello 1983a; 1985; Lopes & Tibana 1987; 1991; Mulieri et al. 2010),
although they are very difficult to identify due to the high similarity and variability observed
in chaetotaxy and genital morphology of close species. For several other species, females are
still unknown or there is a lack of more detailed studies that relate them to males already
described.
Material and Methods
Specimens here studied are deposited in the following institutions:
CE-TdeA – Tecnológico de Antioquia, Institución Universitaria (Medellín, Colombia);
MNRJ – Museu Nacional/Universidade Federal do Rio de Janeiro (Rio de Janeiro, Brazil);
NRM – Swedish Museum of Natural History (Stockholm, Sweden);
ZMUC – Zoological Museum of Copenhagen (Copenhagen, Denmark).
Digital photographs were taken of the lateral habitus, male terminalia in lateral,
anterior (whenever possible) and posterior views for the already described species with
specimens available for examination and also for the newly described species, using a digital
camera Leica DELUX 3™
(10 megapixels), mounted on a Leica S8AP0™
stereoscope or using
a digital camera Carl Zeiss AXIOCAM MRc™
(5 megapixels), mounted on a Carl Zeiss
STEREO DISCOVERY.V12™
stereoscope. For each view, exposures were taken with
extended deep-focus and were stacked using Zerene Stacker™
software. Species for which no
specimens were available for study were documented using information from the literature and
the best illustration of the male terminalia already published were presented after edition.

17
In addition to the study of specimens, a thorough review of the literature about
Oxysarcodexia until December 2013 was performed for compiling a taxonomic list of valid
species, in alphabetical order. Taxonomic data are given with the following structure: valid
name; synonyms; type locality; depository of type specimens; diagnosis; remarks, whenever
relevant; geographical distribution; and, whenever possible, notes about the species biology.
The distributional data were based on the Catalogue of the Sarcophagidae of the World (Pape
1996), on additional records of the literature and on inclusion of localities where specimens
studied in the present conspectus were collected, with the following structure: biogeographic
region, countries and their states/provinces (alphabetically ordered). New records of
occurrence are underlined. All pictorial information is given in plates for each species, in the
appendix, also in alphabetical order.
Depositories of the species are cited by the following acronyms (plus the three
institutions listed previously):
AMNH – American Museum of Natural History, Department of Entomology (New York,
New York, USA);
BMNH – The Natural History Museum, Department of Entomology (London, England,
United Kingdom);
CAS – California Academy of Sciences, Department of Entomology (San Francisco,
California, USA);
DEI – Deutsches Entomologisches Institut, Deutschen Akademie der
Landwirtswissenschaften zu Berlin (Eberswalde, Brandenburg, Germany);
MZUSP – Museu de Zoologia, Universidade de São Paulo (São Paulo, São Paulo, Brazil);
NMW – Naturhistorisches Museum Wien (Vienna, Austria);
SMN – Staatliches Museum für Naturkunde (Stuttgart, Germany);
UCC – University of Concepción (Concepción, Biobio, Chile);
UKaL – University of Kansas, State Biological Survey of Kansas Invertebrate Collection
(Lawrence, Kansas, USA);
UPRG – Universidad Nacional “Pedro Ruiz Gallo”, Departamento de Fitotecnia, Museo de
Entomología (Lambayeque, Lambayeque, Peru);

18
USNM – United States National Museum of Natural History, United States National
Entomological Collection (Washington, D.C., USA).
Terminology follows McAlpine (1981) for external characters and Mello-Patiu & Pape
(2000) and Giroux et al. (2010) for male terminalia. Measurement of the body length was
obtained by including the head (without considering the antennae), thorax (from the neck to
the posterior margin of the scutellum), and abdomen (from the anterior margin of abdominal
tergite 2 to the posterior margin of epandrium) lengths, in order to offset bias caused by any
eventual curvature of the specimen. Type specimens were deposited in the Diptera collections
of the MNRJ, NRM and ZMUC, as pointed out specifically in each description. Label
information of the new species and photographed specimens were transcribed without any
modification; a forward slash (/) was used to separate individual labels, whereas a double
forward slash (//) was used to separate different specimens of a same species. Any necessary
additional comment was given inside brackets and the depository in parenthesis at the end of
transcription label information.
Generic Definition
In order to group some species which were included previously in Sarcophaga genus
only for the absence of a specific taxon, Townsend proposed in 1917 the genus Oxysarcodexia
(Townsend 1917). Dasyproctia Enderlein, 1928, Hybopygia Enderlein, 1928, Apelophyla Hall,
1938 and Xarcophaga Dodge, 1968 are considered synonymous of Oxysarcodexia (Lopes
1946b; Dodge 1966; Lopes 1975c). The type species of this genus is Oxysarcodexia peltata
(Aldrich, 1916). Specific characters for species identification are located almost exclusively in
male terminalia (Lopes 1946b).
Broader studies of this genus based on male adults, including descriptions of new
species, list of names, geographic distribution, new combination of taxa and dichotomous key
were performed by Lopes (1946b), Dodge (1966) and Lopes & Tibana (1987). A catalogue of
Sarcophagidae species of the world provide a list of Oxysarcodexia species and their known
distribution until 1995 (see Pape 1996). The study of tergites 6+7, nowadays recognized as
tergite 7 (T7), of 38 species is the most comprehensive approach for females, classified them
into three different groups based on syntergite form: undivided (with 21 species), partially

19
divided into two plates (with 12 species) or membranous (with 5 species) (Tibana & Mello
1985). Other studies in this way are scarce, remaining only a few descriptions and revisions
scattered in several papers, generally covering only for one species (e.g. Lopes 1938; 1946b;
1973a; Blanchard 1939; Dodge 1956; Mulieri et al. 2010).
Oxysarcodexia is considered a monophyletic genus by the autapomorphy of the lateral
triangular extension of the phallic tube (“tooth-like”) above the vesica. Characters as post-
cranium concave, tergite 5 (T5) entirely yellow, juxta microvillose, and median stylus curved
towards distal end of the phallus are considered homoplasies (Giroux et al. 2010). Character
states as postalar wall setose; male with ctenidium in mid femur of flattened spines; tegula
darkish and basicosta lighter (generally light brownish); male sternite 5 (ST5) deeply cleft
with almost parallel sides, with a few exceptions; penis unsegmented; phallus with three
conducting styli and with the “tooth-like” extension above the vesica; vesica elongated,
conspicuous and always well constituted are pointed out in the literature as generic structures
that, occurring together, allow the correct recognition of this taxon (Lopes 1946b; Dodge
1966; Pape 1996; Carvalho & Mello-Patiu 2008; Silva & Mello-Patiu 2008).
Remarks
Juxta. According to Roback (1954), the juxta is a ventral (i.e. apical) appendage of the
“corpus” (i.e. phallic tube) that can be immovable (i.e. fused to the phallic tube), partially or
completely movable. From this standpoint, Oxysarcodexia, beside Agria Robineau-Desvoidy,
1830, Cistudinomyia Townsend, 1917, Ravinia Robineau-Desvoidy, 1863, Angiometopa
Brauer & Bergenstamm, 1889 and Wohlfahrtia Brauer & Bergenstamm, 1889, is considered
having no developed juxta (Roback 1954). However, a later phylogeny approach of Giroux et
al. (2010) redefined this structure as an apical extension of the posterior side of the
distiphallus, which, even without a visible landmark as structural divisions or a groove, for
example, is considered arising from the base of the median stylus. Therefore, Sarcophaginae
subfamily, and consequently Oxysarcodexia genus, is recognized as having a juxta. Our
examination of different Oxysarcodexia species comes in agreement with this later
proposition.

20
Genus Diagnosis
Oxysarcodexia Townsend, 1917
Apelophyla Hall, 1938
Dasyproctia Enderlein, 1928
Hybopygia Enderlein, 1928
Xarcophaga Dodge, 1968
Male. Total length ranges, in average, from 5 to 12mm.
Head. Fronto-orbital, parafacial and postocular plates generally with gold
microtomentum, usually intense, but sometimes pale and/or with a few silvery shades due to
natural conditions or conservation issues; parafacial with a few darkish short setae; occiput
blackish with silvery microtomentum, black setae in dorsomedial and lateral areas and a few
golden setae in ventromedial area; postocular plates with golden or silvery microtomentum;
frontal vitta darkish with a row of frontal setae present until half high of pedicel, not strongly
divergent, and varying among 7–8 and 12–13; inner vertical seta well-developed, outer
vertical seta not differentiated from postocular setae; ocellar setae smaller than or equal in size
to uppermost frontals; 1 reclinate fronto-orbital seta, with variable length and proclinate seta
absent (only one exception, Oxysarcodexia orbitalis Dodge, 1966); gena and postgena darkish
with golden microtomentum, generally intense, but sometimes pale and both with black setae;
antenna dark brown, first flagellomere with brownish microtomentum; arista darkish brown
and long plumose on basal half or basal ¾ ; palpus dark brown; strong vibrissa.
Thorax. Grayish with 3 black vittae and lightly pale or strong golden microtomentum
sometimes is more intense laterally, at humeral region. Chaetotaxy: acrostichals: 0+1;
dorsocentrals unequally developed: 2–3 well developed and 1–3 smaller + 2 well
differentiated and 1–3 smaller or 3 well differentiated (a small seta among these 3 can be
present); intra-alars: 2+2 (not unusual one of them weaker than the other); supra-alars: 2+3;
postalars: 2; postpronotals: 3; notopleurals: 4 (2 large primaries and 2 smaller subprimaries);
katepisternals: 3 with middle one weaker and inserted slightly below the others; meropleurals:
6–12; postalar wall setose; apical scutellar seta can be present or not; subapical: 1; lateral: 1;
basal: 1; discal: 1; proepisternum always bare; prosternum setose.

21
Wings. Hyaline, black tegula, R1 bare (only one exception, Oxysarcodexia
chaetopygialis (Williston, 1896)), R4+5 setulose in proximal ½, ⅔ or ¾ of distance to r-m,
costal spine not differentiated and third costal sector without ventral setulae.
Legs. color generally blackish, but sometimes brownish or yellowish; fore femur with a
row of setae on dorsal, posterodorsal, and posteroventral surfaces; fore tibia with 1 strong and
3 weaker anterodorsal, 1 dorsal, 1 posterior and 1 posteroventral setae; mid femur with 5
median anterior and 2 pre-apical posterior setae, 5 anteroventral and 6 posteroventral long
setae, ctenidium of flattened spines apically on posteroventral surface; mid tibia with 2
anterodorsal, 1 posterodorsal, 1 posteroventral and 1 anterodorsal setae; hind femur with 1
strong pre-apical dorsal and 1 posterodorsal seta, 1 anteroventral and 1 anterodorsal row of
long setae, and 1 posteroventral row of decreasing size setae up to the distal ⅓, approximately;
hind tibia with 2 anteroventral, one dorsal, 2 posterodorsal and 3 anterodorsal setae;
tarsomeres of fore, mid and hind legs with a weak ventral golden micromentum.
Abdomen. Silvery or yellow-silveryish microtomentum, sometimes with golden
microtomentum more intense on lateral margin of the tergites; tergite 2 (T2) with silvery (most
common) or yellow-silveryish microtomentum; tergite 3 (T3) with 1–3 lateral marginal setae;
tergite 4 (T4) with 1–4 lateral marginal and 0–2 median marginal setae; tergite 5 (T5)
completely yellow and with about 16–23 strong setae along the posterior margin; sternites 2–4
oblong or square with scattered setulae of variable length, generally stronger along the
posterior edge; sternite 5 (ST5) with a median deep cleft of almost parallel edges (only a few
exceptions – 8 species – which have V-shaped edges) forming arms of variable width (thin,
medium, large), microtomentum variable (completely or partially yellow or dark) and with the
presence of window (space between the arms at ventroposterior edge of T5 level) and setosity,
setae or both along the arms, especially along the posteroapical edge.
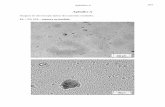
Terminalia (Fig. 1). Syntergosternite 7+8 yellow brownish with golden
microtomentum, scattered short black setulae, and 4–10 marginal well developed setae.
Epandrium is yellowish, generally intense, but sometimes pale or blackish, though still
showing golden microtomentum; presence of black setae. Surstylus triangular (enlarged base
and narrow apex) or oblong (elongated dorsoventrally with base not very enlarged than the
apex) and discal setulae present, although a strong seta not always present. Cercus, in lateral
view, straight, sinuous or bent backwards (more rare), with the apex, which can be darker than

22
the rest of the cercus, pointed, expanded (apical expansion straight, oblique or concave) or
normal (i.e. with the same size as the median area), and with the presence of setae dorsally
(length of setae can be variable) and setosity ventrally; in posterior view, conformation
parallel or divergent, especially at apical portion, a remarkable constriction at the middle
portion of the cercus and a lateromedial pads of setae on the apex can be present in some
species. Postgonite is dark brownish, slender, slightly curved, with apex shape variable
(square, pointed), and sometimes with the presence of a seta. Pregonite is dark brownish,
always broader than postgonite, generally curved and with base and apex of same size or
expanded at the base and narrower at the apex (narrowing abruptly or smoothly); apex shape
variable (sharped, pointed, rounded) and mostly darker (blackish) than the base. Phallus well
developed and sclerotized, without division between basi- and distiphallus (i. e. unsegmented),
with the posterodorsal face more sclerotized than the anteroventral; presence of a lateral
triangular extension of the phallic tube (“tooth-like”) above the vesica; distiphallus
ventroapical margin, in lateral view, smooth or serrated; ventroapical concavity at the
distiphallus can be observed in some species; distiphallic apical shape conic, rounded or
square/oblong; specific structures at the distiphallus, as lateroapical furrow, ventroapical
projections, lateral lobes and dorsoapical membranous formation (“swelling-like”) can be
observed in some species. Median stylus coming towards the phallic tube apex and is
connected by dorsal roads (as named by Roback (1954) and pointed out by Silva & Mello-
Patiu (2008)) to the vesica. Juxta bifurcated, rough laterally, smooth ventrally and joined to
the phallic tube. Presence of a conspicuous and highly modified vesica inserted at
approximately the middle of the ventromedial portion of the phallic tube; a basal branch
comes out from the distiphallic tube and splits mediolaterally into two lateral branches that can
end up in filament (at most tapering to the apex), rounded or square terminal lobes, with
normal or reduced sizes. The vesica is articulated and presents a great variety of
ornamentation. In some species, a median projection with an angular or rounded peak can be
present at the basal branch before the mediolateral division of the vesica. Lateral lobes, i.e.
division of the vesica coming from or close to the basal branch of the vesica, placed laterally
to phallic tube is another vesica structural modification seen in some species. The whole
vesica is well sclerotized, although terminal lobes can be more membranous. Spines can be
present along the edges, only on ventral surface, only on dorsal surface or in both surfaces of

23
terminal lobes. Despite of the great shape variation of the vesica, shape as the “leaf-like”
terminal lobes is one of the most common. In only 8 species vesica is asymmetric, having left
side modified somehow (e.g. reduced, displaced in transverse plane, etc.).
Species awaiting status confirmation
O. aureiceps, O. dorisae, O. flavifrons and O. neivae
Descriptions of O. aureiceps (Macquart, 1855) (and incorrect spelling O. aurescens
(Lopes &Tibana 1987)), O. dorisae Dodge, 1965 (and incorrect spelling O. dorissae (Lopes
&Tibana 1987)), O. flavifrons (Macquart, 1846) and O. neivae Mattos, 1919 are based only on
female specimens. Lopes (1946b) considered O. flavifrons probably synonymy with O. varia
(Walker, 1836), although, in a posterior study (Lopes & Tibana 1987), this species was
ascribed as “inquaerendae”, i.e., with doubtful description. Therefore, for the moment, it is
wiser consider these species lacking status confirmation until a wide and deep study of
females is conducted. Oxysarcodexia aureiceps is considered “inquirenda” for being assigned
to Oxysarcodexia genus although presenting doubtful identity (Lopes & Tibana 1987; Pape
1996) and also presenting very bad conditions of preservation according to Aldrich (1930).
Taxonomy
Oxysarcodexia n. sp. 1
(Appendix 1)
Type locality. ECUADOR: Napo Province.
Depository of type material. ZMUC.
Description. Male. Total length = 8.30–8.50 mm (n=2). Head. Fronto-orbital,
parafacial and postocular plates with gold microtomentum; occiput blackish with silvery
microtomentum, black setae in dorsomedial and lateral areas and a few golden setae in
ventromedial area; front about 0.08x head width at level of ocellar triangle; frontal vitta
darkish with row of 9–11 frontal setae; inner vertical seta well-developed, outer vertical seta
not differentiated; ocellar setae equal in size to uppermost frontals; 1 reclinate fronto-orbital
seta, with variable length (⅓ larger than the frontals or equal in size) and proclinate seta
absent; gena and postgena black, but with, respectively, golden and silvery microtomentum,

24
and both with black setae; antenna dark brown, first flagellomere with brownish
microtomentum and about 2.00x longer than pedicel; arista brown with the middle portion
lighter and long plumose on basal ¾; palpus dark brown. Thorax. Grayish with lightly pale or
stronger golden microtomentum, more intense laterally; chaetotaxy: acrostichals 0+1,
dorsocentrals 3+5, intra-alars 2+2, supra-alars 2+3, postalars 2, postpronotals 3, notopleurals 4
(2 large primaries and 2 smaller subprimaries), katepisternals 3 with the middle one weaker
and inserted slightly below the others, meropleurals 8–9, postalar wall setose, scutellum with 1
apical, 1 subapical, 1 lateral, 1 basal and 1 discal seta; prosternum setose on the ⅔ distal.
Wings. Hyaline, black tegula, R1 bare, R4+5 setulose in proximal ⅔ of distance to r-m, costal
spine not differentiated and third costal sector without ventral setulae. Legs. blackish brown;
fore femur with a row of setae on dorsal, posterodorsal, and posteroventral surfaces; fore tibia
with one strong and 3 weaker anterodorsal, 1 dorsal, 1 posterior and 1 posteroventral setae;
mid femur with 5 median anterior and 2 pre-apical posterior setae, 5 anteroventral and 6
posteroventral long setae, ctenidium of flattened spines apically on posteroventral surface; mid
tibia with 2 anterodorsal, 1 posterodorsal, 1 posteroventral and 1 anterodorsal setae; hind
femur with 1 strong pre-apical dorsal and 1 posterodorsal seta, 1 anteroventral and 1
anterodorsal row of long setae, and 1 posteroventral row of decreasing size setae up to the
distal ⅓, approximately; hind tibia with 2 anteroventral, 1 dorsal, 2 posterodorsal and 3
anterodorsal setae; tarsomeres of fore, mid and hind legs with a weak ventral golden
micromentum. Abdomen. Dark brownish with golden microtomentum; tergite 2 with silvery
microtomentum; T3 with 1 marginal lateral seta; T4 with 1 marginal lateral and 1 median
marginal seta; T5 with about 17–23 strong setae along the posterior margin; sternites 2–4
oblong with scattered setulae, stronger along the edges; ST5 with a median deep cleft, with
edges almost parallel. Terminalia. Syntergosternite 7+8 yellow brownish with golden
microtomentum, scattered short black setulae and 8–10 marginal bristles; epandrium yellowish
with golden microtomentum and black setae; surstylus triangular with enlarged base and
narrow apex and sparse marginal and discal setulae; cercus, in lateral view, straight with a
darker apical expansion and, in posterior view, with apex slightly divergent; postgonite
slender, slightly curved, with a seta on the distal ⅓, approximately, and square apex; pregonite
broader, curved and with sharp apex. Phallus well sclerotized, without division between basi-
and distiphallus; oval distiphallic apex; juxta bifurcated, rough laterally and smooth ventrally;

25
vesica well sclerotized and well developed, with a ventrobasal projection, slightly curved
dorsally and, in anterior view, leaf-like branches with spines in dorsal surface (absent
anteriorly).
Female. Unknown.
Etymology. The specific epithet is given based on the grass leaf-like shape of the
vesica; formed by joint the Latin words agros = plant (grass-like) and frons = leaf.
Remarks. Cerci and phallus shape of Oxysarcodexia n. sp. 1 present similarities to
those seen on Oxysarcodexia amorosa (Schiner, 1868), O. berlai, Oxysarcodexia similata
Lopes & Tibana, 1987 and Oxysarcodexia xanthosoma (Aldrich, 1916). The main differences
among them are observed in the vesica, which in Oxysarcodexia n. sp. 1 is more spinous and
doesn‟t have the same basal portion of terminal lobes as the others, and on distiphallus
ventroapical area, without the presence of a ventroapical cleft, seen in lateral view on O.
amorosa, O. similata and O. xanthosoma.
Distribution. NEOTROPICAL. Colombia (San Martin) and Ecuador (Napo Province).
Biology. Unknown.
Material examined. ♂ [holotype]: ECUADOR: Napo Province: Yasuní National Park:
Yasuní Research Station: 76° 36‟W 00° 38‟S: 3–20 XI 1998: T. Pape & B. Viklund / NRM-
DIPT 0014467 (NRM) // ♂ [paratype]: COLOMBIA: Amazonas PNN Amacayanu, Camino a
San Martin; 3°41‟N 70°15‟W; 1–10.iii.2004, sweepnet; T. Pape & D. Arias. Id# 4325 / NRM-
DIPT 0014650 (NRM).
Oxysarcodexia n. sp. 2
(Appendix 2)
Type locality. FRENCH GUIANA: Montsinery.
Depository of type material. ZMUC.
Description. Male. Total length = 6.8 mm. Head. Fronto-orbital, parafacial and
postocular plates with gold microtomentum; occiput blackish with silvery microtomentum,
black setae in dorsomedial and lateral areas and a few golden setae in ventromedial area; front
about 0.1x head width at level of ocellar triangle; frontal vitta blackish, with row of 9–11
frontal setae; inner vertical seta well-developed, outer vertical seta 0.2x as long as the inner
one and as long as a postocellar seta; ocellar setae about 0.3x as long as the frontal setae; 1

26
reclinate fronto-orbital seta equal in size to frontals and proclinate seta absent; gena and
postgena blackish with silvery microtomentum and black setae; antenna dark brown, first
flagellomere with pale golden microtomentum and about 3.3x as long as pedicel; arista dark
brown with the middle portion lighter and long plumose on basal ⅔; palpus dark brown.
Thorax. Grayish with pale golden microtomentum, slightly more intense laterally; chaetotaxy:
acrostichals 0+1, dorsocentrals 3+5, intra-alars 2+2, supra-alars 2+2, postalar 1, postpronotals
3, notopleurals 4 (2 large primaries and 2 smaller subprimaries), katepisternals 3 with the
middle one weaker and inserted slightly below the others, meropleurals 7, postalar wall setose,
scutellum with 1 apical, 1 subapical, 1 lateral, 1 basal and 1 discal seta; prosternum bare.
Wings. Hyaline, black tegula, R1 bare, R4+5 setulose in proximal ⅔ of distance to r-m, costal
spine small, but distinct and third costal sector without ventral setulae. Legs. blackish brown,
fore femur with a row of setae on dorsal and anteroventral surfaces; fore tibia with 1 dorsal, 1
anterior, 1 anterodorsal and 1 posteroventral setae; mid femur with 5 median anterior and 2
pre-apical posterior setae, ctenidium of flattened spines apically on posteroventral surface, 1
posteroventral and 1 anteroventral row of long setae; mid tibia with 1 anterior, 2 anterodorsal,
1 posterodorsal, 1 anteroventral and 1 posteroventral setae; hind femur with 1 posterodorsal
seta, 1 anteroventral and 1 anterodorsal row of long setae; hind tibia and hind tarsomeres lost
on both sides. Abdomen. Dark brownish with golden microtomentum more intense laterally;
T3–4 with 2 marginal lateral setae; T5 with about 20 strong setae along the posterior margin;
ST2–4 oblong with a pair of strong setae on posterior margin; ST5 with a median deep cleft,
with edges almost parallel. Terminalia. Syntergosternite 7+8 yellow brownish with sparse
golden microtomentum, scattered short black setulae and 9 marginal bristles; epandrium
yellow brownish with sparse golden microtomentum and short black setulae; surstylus
triangular with enlarged base and narrow apex and sparse marginal and discal setulae; cercus,
in lateral view, straight with a darker apical expansion and, in posterior view, with apex
slightly divergent; postgonite slender, slightly curved and with a square apex; pregonite
broader, longer, curved and with pointed apex. Phallus well sclerotized, without division
between basi- and distiphallus; rounded distiphallic apex; juxta with a rugous aspect; vesica
well sclerotized with, in lateral view, median lobes turned on ventroapical direction, tiny
spines along the edges and on dorsal surface and, in anterior view, chicken wing-like
branches.

27
Female. Unknown.
Etymology. The specific epithet refers to the vesica shape in lateral and anterior views,
which reminds chicken wings. From Latin, alectorius is an adjective relative to peacock.
Remarks. Vesica shape is very similar to that observed in Oxysarcodexia angrensis
(Lopes, 1933), although Oxysarcodexia n. sp. 2 present rounded distiphallus apex and cerci
with enlarged apex contrasting with the conic distiphallus apex and cerci with pointed apex of
O. angrensis.
Distribution. NEOTROPICAL. French Guiana (Montsinery).
Biology. Unknown.
Material examined. ♂ [holotype]: FRENCH GUIANA: Montsinery; “Emerald
Jungle”; Carrefour du Gallion; 01.i.2003, M. Kotrba / NRM-DIPT 0014601 (NRM).
Oxysarcodexia n. sp. 3
(Appendix 3)
Type locality. COSTA RICA: Puntaneras, Monteverde.
Depository of type material. ZMUC.
Description. Male. Total length = 9.5–10.0 mm (n=3). Head. Fronto-orbital,
parafacial and postocular plates with gold microtomentum; occiput blackish with silvery
microtomentum, black setae in dorsomedial and lateral areas and golden setae in ventromedial
area; front about 0.1x head width at level of ocellar triangle; frontal vitta dark, slight brownish
anteriorly, with row of 7–9 frontal setae; inner vertical seta well-developed, outer vertical seta
not differentiated; ocellar setae equal in size to uppermost frontals; 2 reclinate fronto-orbital
setae equal in size to frontals and proclinate seta absent; gena and postgena black, but with,
respectively, golden and silvery microtomentum, and both with black setae; antenna dark
brown, first flagellomere with brownish microtomentum and about 2.3x longer than pedicel;
arista dark brown with the middle portion lighter and long plumose on basal ¾; palpus dark
brown. Thorax. Grayish with lightly pale golden microtomentum; chaetotaxy: acrostichals
0+1, dorsocentrals 2+4, intra-alars 2+2, supra-alars 3+3, postalars 2, postpronotals 3,
notopleurals 4 (2 large primaries and 2 smaller subprimaries), katepisternals 3 with the middle
one weaker and inserted slightly below the others, meropleurals 10, postalar wall setose,
scutellum with no apical, 1 subapical, 1 lateral and 1 discal seta; prosternum setose at edges.

28
Wings. Hyaline, black tegula, R1 bare, R4+5 setulose in proximal ⅓ of distance to r-m, costal
spine not differentiated and third costal sector without ventral setulae. Legs. blackish brown,
fore femur with a row of setae on dorsal, posterodorsal, and posteroventral surfaces; fore tibia
with 2 anterodorsal, 1 posterior and 1 posteroventral setae; mid femur with 5 median anterior
and 3 pre-apical posterior setae, ctenidium of flattened spines apically on posteroventral
surface, 1 posteroventral and 1 anteroventral row of long setae; mid tibia with 1 anterior, 2
posteroventral and 1 posterodorsal setae; hind femur with a strong anteroventral and a pre-
apical posterior seta, 1 posteroventral and 1 anterodorsal row of long setae; hind tibia with 1
anterior row of long setae, 2 anteroventral, 1 dorsal, 3 posterodorsal and 2 posterior setae;
tarsomeres of fore, mid and hind legs with a ventral golden micromentum, weaker in mid legs.
Abdomen. Blackish gray with silvery microtomentum more intense laterally; T3 with 2
marginal lateral setae; T4 with 3 marginal lateral setae; T5 with about 18 strong setae along
the posterior margin; ST2–4 square with a pair of strong setae on posterior margin; ST5 with a
median deep V-shaped cleft. Terminalia. Syntergosternite 7+8 large, brownish with golden
microtomentum laterally, scattered short black setulae and 12 marginal bristles; epandrium
yellowish with short black setulae and golden microtomentum; surstylus oblong with narrow
base and apex, enlarged on ⅔ portion, with sparse marginal and discal setulae and a long and
slender colorless setae at apex; cercus, in lateral view, slightly sinuous with blackish preapical
expansion and pointed apex; postgonite slender and slightly curved, especially at the apex;
pregonite broad with narrower rounded apex. Phallus well sclerotized, without division
between basi- and distiphallus; juxta bifurcated with small spines along the inner edges, except
at the apex; 2 lateral styli and 1 median stylus both tubular-shaped and partially hidden by the
juxta; vesica well sclerotized and well developed, with an angular ventroapical projection
about 1.2 mm longer than distiphallic apex and, in anterior view, enlarged base and bifurcated
apex with small spines and horseshoe-like branches.
Female. Unknown (see remarks).
Etymology. The specific epithet is related to the right angle seen on vesica
conformation. From the Latin, angulosus = with corners or angles.
Remarks. Cerci of Oxysarcodexia perneta (Walker, 1861) is similar to that found on
Oxysarcodexia n. sp. 3. However, Oxysarcodexia n. sp. 3 terminalia constitution is very
peculiar, especially for the vesica shape, not found in any other species of the genus. A female,

29
labelled “COSTA RICA, San José; Cerro Muerte, 16km S; Empalme, 2600m; III–VI 1990
Hanson / COSTA RICA INBIO; CRI001; 107347” is mostly likely the female of this species
because of the blackish occiput with silvery microtomentum, black setae in dorsomedial and
lateral areas and, even sparser than in male, golden setae in ventromedial area and the body
color pattern (grayish thorax lightly pale with golden microtomentum, abdomen blackish gray
with silvery microtomentum and genital segments yellowish with golden microtomentum)
very similar to those found in males. Nevertheless, without a more exhaustive study of female
comparative morphology we prefer to exclude it from “formal type status”.
Distribution. NEOTROPICAL. Costa Rica (Puntaneras, San José, and Punta).
Biology. Unknown.
Material examined. ♂♂ [holotype]: COSTA RICA: Punt. Monteverde 1840m; Cerro
Amigos; 14.VI.2000, T. Pape / INB0003086811 (ZMUC) // [paratype]: Est, Cuerici. Send. El
Carbon, 4.6km al E. de Villa Mills, Prov. San Jose, COSTA RICA 2600m. 26–31 OCT / 1995.
B. Gamboa, L_S_389550_ / 500050 #6326 / COSTA RICA INBIO; CRI002; 363463 (ZMUC)
// [paratype]: Estac. Pitier, Prov. Punta, COSTA RICA. 1679m. 5–18 ENE 1995. G. Fonseca,
L_N_330900_577400 #5950 (ZMUC).
Oxysarcodexia n. sp. 4
(Appendix 4)
Type locality. BRAZIL: Rio de Janeiro.
Depository of type material. MNRJ.
Description. Male. Total length = 7.7 mm. Head. Fronto-orbital, parafacial and
postocular plates with golden microtomentum; occiput blackish with golden microtomentum,
black setae in dorsomedial and lateral areas and a few golden setae in ventromedial area; front
about 0.1x head width at level of ocellar triangle; frontal vitta blackish, with a row of 14
frontal setae; inner vertical seta well-developed, outer vertical seta 0.3x as long as the inner
one; ocellar setae 0.8x as long as the uppermost frontal setae; 1 reclinate fronto-orbital seta
2.2x as long as the uppermost frontal and proclinate seta absent; gena and postgena blackish
with golden microtomentum and black setae; antenna dark brown, first flagellomere with pale
golden microtomentum and about 2.3x as long as pedicel; arista brown and long plumose on
basal ⅔; palpus dark brown. Thorax. Greyish with golden microtomentum; chaetotaxy:

30
acrostichals 3+1, dorsocentrals 3 (first weaker) + 3, intra-alars 2+2, supra-alars 2+3, postalars
2, postpronotals 3, notopleurals 4 (2 large primaries and 2 smaller subprimaries),
katepisternals 3 with the middle one weaker and inserted slightly below the others,
meropleurals 8–9, postalar wall setose, scutellum with 1 apical, 1 subapical, 1 lateral, 1 basal
and 1 discal seta; prosternum with few setae along the edges. Wings. Hyaline, black tegula,
R1 bare, R4+5 setulose in proximal ¾ of distance to r-m, costal spine not differentiated and
third costal sector without ventral setulae. Legs. darkish brown, fore femur with 2 rows of
setae on posterodorsal and 1 on posteroventral surfaces; fore tibia with 3 dorsal, 1
posterodorsal, 2 posteroventral and 1 posterior setae; mid femur with 6 median anterodorsal, 2
pre-apical posterior setae, 1 anteroventral and 1 posteroventral row of setae and ctenidium of
flattened spines apically on posteroventral surface; mid tibia with 1 dorsal, 2 posterodorsal, 1
posterior, 1 posteroventral, 1 ventral, 1 anterior and 2 anterodorsal setae; hind femur with 1
posterodorsal and 1 dorsal seta, 1 anteroventral and 1 anterodorsal row of long setae, 1 apical
posteroventral row of decreasing setae and 1 anterior row of decreasing setae; hind tibia with 2
anteroventral, 3 anterodorsal, 2 dorsal, 1 anterior and 2 posterodorsal setae; tarsomeres of fore
and hind legs with a weak ventral golden micromentum. Abdomen. Grayish with silvery
microtomentum; T2 with 1 marginal lateral seta, T3 with 2 marginal lateral setae and T4 with
4 marginal lateral and 1 median marginal setae; T5 with about 20 strong setae along the
posterior margin; ST2–4 oblong with scattered setulae on posterolateral and posterior edges;
ST5 with a median deep cleft with almost parallel arms. Terminalia. Syntergosternite 7+8
dark brownish with golden microtomentum and scattered black setulae and 8 marginal bristles;
epandrium dark brownish with golden microtomentum, short black setulae and 2 strong setae
dorsoapically; surstylus oblong, elongated dorsoventrally, with sparse marginal and discal
black setulae; cercus, in lateral view, with a basal expansion, square and slightly curved apex;
postgonite slender, slightly curved apically and with square apex; pregonite broader, slightly
curved and rounded apex. Phallus sclerotized, without division between basi- and distiphallus;
distiphallic apex oblique, with lateral, dorsal and ventral projections, smooth edges and a wide
opening ventroapically; vesica well sclerotized, coming out pre-apically from the phallic tube
as a short branch that fold vetroposteriorly culminating in flattened elongated lobes with
microspines on dorsal surface.
Female. Unknown.

31
Etymology. The specific epithet is given in honor of Ariozano de Souza, father of the
first author.
Remarks. Presence of enlarged apex of the phallus as observed in Oxysarcodexia n.
sp. 4, Oxysarcodexia favorabilis (Lopes, 1935), Oxysarcodexia fraterna (Lopes, 1946),
Oxysarcodexia n. sp. 5, Oxysarcodexia nitida Soares & Mello-Patiu, 2010, Oxysarcodexia
notata Soares & Mello-Patiu, 2010, Oxysarcodexia pallisteri Dodge, 1966, Oxysarcodexia
peruviana (Lopes, 1973), Oxysarcodexia vittata (Lopes, 1946) and Oxysarcodexia xon
(Dodge, 1968) (all previously considered as belonging to “Xarcophaga group” and remarked
by Soares & Mello-Patiu (2010), except for Oxysarcodexia n. sp. 4, O. fraterna,
Oxysarcodexia n. sp. 5 and O. peruviana which we included in this group for presenting the
same characteristic). Vesica with “flower-like” shape is another similarity seen on
Oxysarcodexia n. sp. 4, O. favorabilis and Oxysarcodexia n. sp. 5.
Distribution. NEOTROPICAL. Brazil (Rio de Janeiro).
Biology. Unknown.
Material examined. ♂ [holotype]: BRAZIL: RJ Tijuca Forest nr Rio; 7–30.ix.1993,
T.Pape / NRM-DIPT 0014641 (MNRJ).
Oxysarcodexia n. sp. 5
(Appendix 5)
Type locality. ECUADOR: Napo Province
Depository of type material. ZMUC.
Description. Male. Total length = 6.0 mm. Head. Fronto-orbital, parafacial and
postocular plates with pale golden microtomentum; occiput blackish with silvery
microtomentum, black setae in dorsomedial and lateral areas and a few golden setae in
ventromedial area; front about 0.1x head width at level of ocellar triangle; frontal vitta
blackish, with a row of 8–9 frontal setae; inner vertical seta well-developed; outer vertical seta
not differentiated; ocellar setae 1.3x as long as the uppermost frontal setae; 1 reclinate fronto-
orbital seta 2.3x as long as the uppermost frontal and proclinate seta absent; gena and postgena
blackish with pale golden microtomentum and black setae; antenna dark brownish, first
flagellomere with pale golden microtomentum and about 2.2x as long as pedicel; arista brown
and long plumose on basal ¾; palpus dark brown. Thorax. Greyish with pale golden

32
microtomentum; chaetotaxy: acrostichals 0+1, dorsocentrals 3 (first weaker) + 3, intra-alars
2+2, supra-alars 2+3 (middle one stronger), postalars 2, postpronotals 3 (the lateromedial one
weaker), notopleurals 4 (2 large primaries and 2 smaller subprimaries), katepisternals 3 with
the middle one weaker and inserted slightly below the others, meropleurals 9, postalar wall
setose, scutellum without apical, 1 subapical, 1 lateral (weaker), 1 basal and 1 discal seta;
prosternum with few setae along the edges. Wings. Hyaline, black tegula, R1 bare, R4+5
setulose in proximal ⅔ of distance to r-m, costal spine not differentiated and third costal sector
without ventral setulae. Legs. darkish brown, fore femur with a row of setae on posterodorsal
and posteroventral surfaces, and a row of decreasing setae on posterior surface; fore tibia with
1 dorsal, 2 posterodorsal, 1 anterodorsal and 1 anterior setae; mid femur with 5 anterodorsal, 2
pre-apical posterior, 4 anteroventral, 4 posteroventral setae and ctenidium of flattened spines
apically on posteroventral surface; mid tibia with 2 posterodorsal, 1 posteroventral, 1 ventral,
1 anteroventral and 2 anterodorsal setae; hind femur with 1 posterodorsal and 1 dorsal seta, 1
anteroventral and 1 anterodorsal row of long setae, 1 apical posteroventral row of decreasing
setae; hind tibia with 1 anteroventral, 3 anterodorsal, 1 dorsal and 2 posterodorsal setae;
tarsomeres of fore, mid and hind legs with a weak ventral golden micromentum. Abdomen.
Grayish with pale golden microtomentum; T2 with silvery microtomentum laterally; T2–3
with 1 marginal lateral seta; T4 with 3 marginal lateral and 1 median marginal setae; T5 with
about 18 strong setae along the posterior margin; ST2–4 square with scattered setae; ST5 with
a median deep cleft with almost parallel arms. Terminalia. Syntergosternite 7+8 yellow
brownish with golden microtomentum and scattered short black setulae and 8 marginal
bristles; epandrium yellow brownish with golden microtomentum, one strong dorsoapical seta
and short black setulae; surstylus triangular with enlarged base and sparse marginal black
setulae; cercus, in lateral view, sinuous with pointed apex; postgonite slender, curved apically
and with pointed apex; pregonite broader, slightly curved and rounded apex. Phallus
sclerotized, flower-like shaped, without division between basi- and distiphallus; distiphallic
apex expanded, with lateral and ventral projections, smooth edges and wide opening apically;
vesica sclerotized, flattened, oblong, coming out medially from the phallic tube and with
microspines ventroapically.
Female. Unknown.

33
Etymology. The specific epithet is given in honor of Maria José Maia de Souza,
mother of the first author.
Remarks. See “remarks” section on Oxysarcodexia n. sp. 4.
Distribution. NEOTROPICAL. Ecuador (Napo).
Biology. Unknown.
Material examined. ♂ [male holotype]: ECUADOR: Napo Province: / Yasuní
National Park: / Yasuní Research Station: / 76° 36‟W 00° 38‟S: 3–20 / XI 1998: T. Pape & B.
Viklund // NRM-DIPT / 0014490 (NRM).
Oxysarcodexia n. sp. 6
(Appendix 6)
Type locality. ECUADOR: Napo Province.
Depository of type material. ZMUC.
Description. Male. Total length = 7.50 mm. Head. Fronto-orbital, parafacial and
postocular plates with pale gold microtomentum; occiput blackish with silvery
microtomentum, black setae in dorsomedial and lateral areas and a few golden setae in
ventromedial area; front about 0.06x head width at level of ocellar triangle; frontal vitta
blackish, with row of 10–11 frontal setae; inner vertical seta well-developed, outer vertical
seta 0.43x as long as the inner one and as long as a postocellar seta; ocellar setae equal in size
to uppermost frontals; 1 reclinate fronto-orbital seta 1.6x as long as than the frontals and
proclinate seta absent; gena and postgena blackish with golden and silvery microtomentum,
respectively, and black setae; antenna blackish, first flagellomere with pale golden
microtomentum and about 2.00x longer than pedicel; arista dark brown and long plumose on
basal ¾; palpus dark brown. Thorax. Greyish with pale golden microtomentum slightly more
intense on the sides; chaetotaxy: acrostichals 0+1, dorsocentrals 4 (first and third weaker) + 4
(first and second weaker), intra-alars 2+2, supra-alars 2+3, postalars 2, postpronotals 3,
notopleurals 4 (2 large primaries and 2 smaller subprimaries), katepisternals 3 with middle one
weaker and inserted slightly below the others, meropleurals 7–8, postalar wall setose,
scutellum with 1 apical, 1 subapical, 1 lateral, 1 basal and 1 discal seta; prosternum with few
setae along the edges. Wings. Hyaline, black tegula, R1 bare, R4+5 setulose in proximal ⅔ of
distance to r-m, costal spine not differentiated and third costal sector without ventral setulae.

34
Legs. dark brownish, fore femur with a row of setae on dorsal, posterodorsal and anteroventral
surfaces; fore tibia with 1 dorsal, 2 anterodorsal, 2 posterodorsal and 1 posteroventral setae;
mid femur with 4 median anterior and 2 pre-apical posterior setae, 4 anteroventral and 4
posteroventral long setae, ctenidium of flattened spines apically on posteroventral surface; mid
tibia with 2 posterodorsal, 1 posterior, 1 posteroventral, 1 anteroventral and 1 anterodorsal
setae; hind femur with 1 dorsal, 1 posterior and 7 anterodorsal setae, 1 anteroventral and 1
anterodorsal row of long setae, 1 apical posteroventral row of decreasing setae; hind tibia with
1 anterior, 2 anteroventral, 3 anterodorsal, 1 dorsal and 2 posterodorsal setae; tarsomeres of
fore and hind legs with a weak ventral golden micromentum. Abdomen. Brownish with
golden microtomentum; tergite 2 with silvery microtomentum; T3 with one marginal lateral
seta; T4 with 1 marginal lateral and 1 median marginal seta; T5 with about 24 strong setae
along the posterior margin; ST2–4 square with a pair of strong setae on posterior margin and
scattered setulae; ST5 with a median deep cleft, with edges almost parallel. Terminalia.
Syntergosternite 7+8 yellow brownish with golden microtomentum and scattered short black
setulae and 8 marginal bristles; epandrium yellow brownish with sparse golden
microtomentum and short black setulae; surstylus oblong, elongated ventrodorsally with
sparse marginal and discal setulae and a long and slender colorless setae at apex;
cercusstraight with a darker apical expansion, in lateral view, andwith apex slightly divergent,
in posterior view; postgonite slender, slightly curved and with pointed apex; pregonite
broader, curved and pointed apex. Phallus well sclerotized, without division between basi- and
distiphallus; distiphallic apex curved ventrodorsally in an obtuse angle; juxta bifurcated,
rugous laterally, smooth ventrally and knurled apically; vesica well sclerotized, small, knurled
along the edges and, in anterior view, with short and almost flat triangular branches.
Female. Unknown.
Etymology. The specific epithet is given based on conformation of the vesica and
distiphallus, which is clearly separated and, in lateral view, give the impression of a cleft
presence. The word rima, in Latin, means cleft, slit.
Remarks. Oxysarcodexia n. sp. 6 is also very much alike to Oxysarcodexia admixta
(Lopes, 1933); Oxysarcodexia n. sp. 6 differences are noticed on distiphallus median margin
of serrated aspect; on more angulated distiphallus apical shape and on vesica longer
posteroanteriorly, reaching distiphallus ventroapical margin.

35
Biology. Unknown.
Distribution. NEOTROPICAL. Ecuador (Napo).
Material examined. ♂ [male holotype]: ECUADOR: Napo Province: Yasuní National
Park: Yasuní Research Station: 76° 36‟W 00° 38‟S: 3–20 XI 1998: T. Pape & B. Viklund /
NRM-DIPT 0014488 (NRM).
Oxysarcodexia admixta (Lopes, 1933)
Sarcophaga admixta Lopes, 1933
(Appendix 7-A)
Type locality. Brazil, Rio de Janeiro, Angra dos Reis. Lopes, 1933: 156.
Depository of type material. MNRJ.
Diagnosis. Male. Postocular plate with silvery microtomentum. Ocellar setae well-
developed. Thorax generally with golden microtomentum, sometimes more intense on
humeral region, contrasting with the silvery microtomentum of the abdomen. Two well
differentiated + 2 smaller dorsocentral post-sutural setae; apical scutellar seta present. Legs
blackish. T3 with 1 lateral marginal seta. T4 with 1 median marginal and 2 lateral marginal
setae. T5 with golden microtomentum only laterally. ST5 with parallel cleft edges and setosity
on the arms. In lateral view, cerci with straight shape, apex expanded with oblique upwards
edge and presence of setae in full ventral extension. Apical shape of the cerci (i.e. last ⅓
portion of the cercus), in posterior view, smaller than the middle part. Cerci conformation
(posterior view) divergent. In lateral view, ventroapical margin of distiphallus smooth; apical
shape of distiphallus conic; dorsal shape straight. Pregonite with same color (brownish) along
the entire extension and with expanded base narrowing smoothly until the apex, same as
postgonite. Vesica with symmetrical branches; terminal lobes reduced, filamentous at most
tapering to apex, with sclerotized texture; presence of spines only along the edges and
presence of a rounded median projection of the main vesica branch.
Remarks. Cerci and general shape of the phallus are very similar to Oxysarcodexia
carvalhoi Lopes, 1946; differences are seen basically on the apex of distiphallus, without a
ventroapical concavity in O. admixta, and on the vesica, more developed in O. carvalhoi,
although still reduced in comparison to other species of the genus. Oxysarcodexia admixta
also presents similarities on terminalia, especially on distiphallus apex (rounded shape) and on

36
vesica (spinous and with apical triangular apex on terminal lobes), in comparison to
Oxysarcodexia avuncula (Lopes, 1933), Oxysarcodexia berlai Lopes, 1975, O. carvalhoi,
Oxysarcodexia diana (Lopes, 1933) and Oxysarcodexia ventricosa (Wulp, 1895) (species
included in “ventricosa group”, according to Lopes (1975c), that considers also morphology of
females for grouping them). See also “remarks” section of Oxysarcodexia n. sp. 6. Female is
classified by Tibana & Mello (1985), according to the shape of the T6+7, as syntergite
undivided.
Distribution. NEOTROPICAL. Argentina (Misiones), Brazil (Distrito Federal, Goiás,
Mato Grosso, Mato Grosso do Sul, Minas Gerais, Paraná, Rio de Janeiro, Santa Catarina, São
Paulo).
Biology. It is reported in the literature the attractiveness of O. admixta by human feces,
fish, mouse and pig carcasses, chicken viscera (especially rotting liver), marine animals,
banana plus brown sugar and rotten Syagrus comosa (Mart.) Mart. (Arecaceae), a species of a
palmtree (at coastal area) (Lopes 1973b; Dias et al. 1984c; Mendes & Linhares 1993;
D‟Almeida & Lima 1994; Oliveira et al. 2002; Vairo et al. 2011). This fly has already been
found rearing on human feces and mouse carcass (Lopes 1973b; D‟Almeida 1994), and on
agar plus powder milk for 24h then transferred to meat, under laboratory conditions (Lopes
1973b). At our laboratory, it was reared on minced bovine meat, although larviposition was
observed also on rotten fish, with adults emerging after 15–19 days (6 days from larvae to
pupa stage and 9–13 days until adult emergence). This species was also associated to the gum
of Terminalia argentea Mart & Zucc (Combretaceae), a Brazilian pioneer tree, collecting or,
sometimes, ingesting its exudate, probably due to the high concentration of complex
carbohydrates present in this resource (Boff et al. 2008).
Material examined. ♂♂: BRAZIL: São Paulo, Jundiaí, edge of Serra do Japi;
28.X.2011; A. G. Savino (L2B-DBA) // BRAZIL: São Paulo, Jundiaí, Serra do Japi;
24.I.2012; M. D. Grella (L2B-DBA).
Oxysarcodexia adunca Lopes, 1975
(Appendix 7-B)
Type locality. Brazil, Espírito Santo, Conceição da Barra. Lopes 1975c: 475.
Depository of type material. MNRJ.

37
Diagnosis. Male. Postocular plate with pale golden microtomentum. Ocellar setae
smaller than the superior frontal seta. Thorax gray, sometimes yellowish gray, especially at
humeral region. Two well differentiated + 2 smaller dorsocentral post-sutural setae; apical
scutellar seta absent. Legs blackish. Abdomen with silvery microtomentum, although golden
microtomentum is observed laterally on T4 and on entire extension of T5. T3 with 1 lateral
marginal seta. T4 with 1 median marginal and 1 lateral marginal setae. T5 with golden
microtomentum along the entire extension. ST5 with parallel cleft edges, presence of setosity
and setae at the apex of the arms. Cerci, in lateral view, sinuous with expanded apexes of
oblique upwards edges and darker than the median and basal portions. Setae ventrally on the
cerci (lateral view) absent only in middle portion. Apical shape of the cerci (last ⅓ portion of
the cercus; posterior view) with the same size as the middle part. Conformation of the cerci in
posterior view is divergent. In lateral view, ventroapical margin of distiphallus smooth;
distiphallic apical shape rounded; dorsal shape straight. Remarkable presence of microspines
at distiphallus apex. Pregonite with expanded base and sudden narrowing at apex which is
darker; same as postgonite, except for the color, which is the same in full extension. Vesica
with symmetrical branches; terminal lobes well-developed, rounded, with membranous
texture, presence of spines only on ventral surface and presence of an angular median
projection of the main vesica branch.
Remarks. This species presents similar cerci as Oxysarcodexia cyaniforceps (Hall,
1933), but differs by the apical distiphallus shape, pilose in O. adunca, and by the constitution
of the vesica (Lopes 1975c).
Distribution. NEOTROPICAL. Brazil (Bahia, Espírito Santo, Rio de Janeiro),
Ecuador (Napo Province).
Biology. Unknown.
Material examined. ♂♂: Linhares, Espírito Santo, Brasil / P. C. Elias; VI-72 /
paratypus / Oxysarcodexia adunca; n. sp.; ♂; Det. H. S. Lopes / NRM-DIPT 0014213 (NRM)
// ECUADOR: Napo Province, Yasuní National Park, Yassuní Research Station, 76°36‟W
00°38‟S; 3–20.XI.1998; T. Pape & B. Viklund / NRM-DIPT 0014473 (NRM).
Oxysarcodexia afficta (Wulp, 1895)
Sarcophaga afficta Wulp, 1895

38
(Appendix 7-C)
Type locality. Mexico, Morelos, Cuernavaca; Mexico, Veracruz, Atoyac; México,
Yucatán. Wulp, 1895: 269.
Depository of type material. BMNH.
Diagnosis. Male. Postocular plate with pale golden microtomentum. Ocellar setae not
well-developed. Thorax generally with grayish microtomentum. Dorsocentral post-sutural
setae with 2 well differentiated and 1–3 anterior setae smaller. Apical scutellar seta absent.
Legs blackish. Abdomen grayish with shades of pale golden microtomentum laterally. T3 with
2 lateral marginal setae. T4 with 2 median marginal and 2 lateral marginal setae. T5 with
golden microtomentum only laterally. ST5 with parallel cleft edges and setosity on the arms.
Cerci, in lateral view, sinuous with expanded apexes of oblique upwards edges. Setae ventrally
on the cerci (lateral view) present in basal half. Apical shape of the cerci (last ⅓ portion of the
cercus; posterior view) with the same size as the middle part. Conformation of the cerci in
posterior view is divergent. In lateral view, ventroapical margin of distiphallus smooth;
distiphallic apical shape conic; dorsal shape of distiphallus straight, and presence of a small
dorsoapical swelling. Pregonite with expanded base narrowing smoothly until the apex, which
is darker; same as postgonite, except for the color, which is the same in full extension. Vesica
with symmetrical branches; terminal lobes well-developed, filamentous at most tapering to
apex, with sclerotized texture and presence of spines only on ventral surface.
Remarks. Oxysarcodexia afficta is considered close to Oxysarcodexia conclausa
(Walker, 1861), Oxysarcodexia thornax (Walker, 1849) and Oxysarcodexia timida (Aldrich,
1916), however easily separated by the unalike vesica shape (Lopes 1946b). Female was
classified by Tibana & Mello (1985), according to the shape of the T6+7, as syntergite
partially divided into two plates.
Distribution. NEARTIC. Mexico (Morelos, San Luis Potosí). NEOTROPICAL.
Colombia, Costa Rica, Ecuador, Mexico (Chiapas, Jalisco, Sinaloa, Veracruz, Yucatán),
Panama.
Biology. Unknown. Information available is only about the collection of the holotype,
using traps for collecting fruit flies (Lopes 1946b).

39
Material examined. ♂♂: N. Perucho; (Otavalo) Ecuador; 200m. I-1971; L. E. Peña
col. / Oxysarcodexia afficta (Wulp); ♂; Det. H. S. Lopes / NRM-DIPT 0014214 (NRM) //
Mexico, VU94; Chiapa de Corzo; COMEXA, 09.11.2010; A. Grzywack, leg (ZMUC).
Oxysarcodexia amorosa (Schiner, 1868)
Sarcophaga amorosa Schiner, 1868
(Appendix 8-A)
Type locality. Brazil. Schiner, 1868: 314.
Depository of type material. NMW.
Diagnosis. Male. Postocular plate with pale golden microtomentum. Ocellar setae not
well-developed. Thorax with golden microtomentum, as well as the abdomen, which is more
intense on T5. Dorsocentral post-sutural setae with 2 well differentiated and 1–3 anterior setae
smaller. Apical scutellar seta present. Legs brownish. T3 with 3 lateral marginal setae. T4 with
1 median marginal and 2 lateral marginal setae. T5 with golden microtomentum in part. ST5
with parallel cleft edges and setosity on the arms. Cerci, in lateral view, straight with expanded
apexes of oblique upwards edges. Setae ventrally on the cerci (lateral view) in full extension.
Apical shape of the cerci (last ⅓ portion of the cercus; posterior view) smaller than the middle
part. Conformation of the cerci in posterior view is divergent. In lateral view, distiphallus
presents ventroapical concavity, with smooth margins; distiphallic apical shape rounded; and
dorsal shape of distiphallus straight. Pregonite with the same color along the entire extension
and with expanded base narrowing smoothly until the apex. Postgonite with expanded base
and sudden narrowing at apex. Vesica with symmetrical branches; terminal lobes well-
developed, filamentous at most tapering to apex, with sclerotized texture, spines on ventral
surface, presence of lateral lobes (i.e. division of the vesica coming from or close to the basal
branch of the vesica, placed laterally to phallic tube) and presence of a rounded median
projection of the main vesica branch.
Remarks. Phenotype variability in different male populations has already been
reported in literature (Lopes 1973b). Oxysarcodexia inflata Lopes, 1975 is considered very
close related to O. amorosa, especially by the color, but the differences are recognized on
distiphallus apex and on vesica conformation (Lopes 1975c). Differences on O. similata
terminalia, in comparison to O. amorosa, are the presence of a slender anteroventral projection

40
directed apically (lateral view) and the large and less oblique internal margins of apical plates
(ventral view) on the distiphallus, and, on vesica constitution, the enlarged and rounded basal
portion, with the presence of spines. Apical plates of O. xanthosoma differs from the
previously mentioned by the serrated edge (Lopes & Tibana 1987). See “remarks” section of
Oxysarcodexia n. sp. 1 for further discussion. Female was classified by Tibana & Mello
(1985), according to the shape of the T6+7, as syntergite partially divided into two plates.
Distribution. NEARTIC. Mexico (San Luis Potosí, Sonora). NEOTROPICAL. Brazil
(Amapá, Amazonas, Bahia, Ceará, Mato Grosso, Mato Grosso do Sul, Minas Gerais, Rio de
Janeiro, Roraima, Santa Catarina, São Paulo), Colombia (Antioquia), Costa Rica, Ecuador,
Guyana, Mexico (Jalisco), Panama (Barro Colorado Island), Peru (Tingo Maria).
Biology. Oxysarcodexia amorosa has been reared on feces of different animals as dog,
felid and human (Lopes 1973b; D‟Almeida 1994), on dead mammal not specified (Dodge
1968) and on fish head and bones (Lopes 1973b). This species has already been reared
successfully at laboratory (Lopes 1973b) and on dead shrimp under non-natural conditions
(D‟Almeida 1989). Using curdled milk as food resource, larvae reared in 3 days and the adults
emerged after 12 days (Lopes 1973b). This fly has shown developmental preference by low
humidity, being found near to dwellings (Lopes 1973b). Attractiveness has been reported to
human and other vertebrates feces, fish, crab, marine animals, rotten Syagrus comosa (Mart.)
Mart. (Arecaceae), a species of a palmtree and rotten banana plus brown sugar (at coastal
areas), chicken viscera, rat carcass, rotting liver, rotting beef lung and squid (Lopes 1973b;
Lopes 1975a; Mendes & Linhares 1993; Pamplona et al. 2000; Oliveira et al. 2002; Sousa et
al. 2011; Ramírez-Mora et al. 2012). Larva of O. amorosa has also been reported involved in
a case of auricular infestation in São João de Meriti, Rio de Janeiro (Figueiredo et al. 2002),
although this species is not recognized as a causing-myiasis agent.
Material examined. ♂♂: Angra dos Reis; E. do Rio; Brasil / Det. H. S. Lopes;
10.10.72 / NRMDIPT 0014218 (NRM) // Angra dos Reis, E. do Rio, Brasil / H. S. Lopes
6.X.71 / Oxysarcodexia amorosa (Schiner) Det. H. S. Lopes ♂.
Oxysarcodexia angrensis (Lopes, 1933)
Sarcophaga angrensis Lopes, 1933
Sarcophaga articulata Hall, 1933

41
Sarcophaga kartabo Curran & Walley, 1934
(Appendix 8-B)
Type locality. Brazil, Rio de Janeiro, Angra dos Reis. Lopes, 1933: 153.
Depository of type material. MNRJ.
Diagnosis. Male. Postocular plate with golden microtomentum. Ocellar setae well-
developed. Thorax grayish with pale golden microtomentum, as well as abdomen in which
golden microtomentum is more evident laterally. Dorsocentral post-sutural setae with 2 well
differentiated and 1–3 anterior setae smaller. Apical scutellar seta absent. Legs blackish. T3
with 3 lateral marginal setae. T4 with 1 median marginal and 2 lateral marginal setae. Golden
microtomentum at entire extension of T5. ST5 with parallel cleft edges with setosity and setae
on the apex of the arms. Cerci, in lateral view, sinuous with pointed apexes of oblique
upwards edges. Setae ventrally on the cerci (lateral view) absent only in middle portion.
Apical shape of the cerci (last ⅓ portion of the cercus; posterior view) with the same size as
the middle part. Conformation of the cerci in posterior view is parallel. In lateral view,
presence of ventroapical concavity on the distiphallus with smooth margins; distiphallic apical
shape conic; dorsal shape of distiphallus sinuous, and presence of a small dorsoapical
swelling. Pregonite with expanded base narrowing smoothly until the apex, which is darker;
same as postgonite, except for the color, which is the same in full extension. Vesica with
symmetrical branches; terminal lobes well-developed, filamentous at most tapering to apex,
with sclerotized texture, presence of spines on both ventral and dorsal surfaces and presence of
an angular median projection of the main vesica branch.
Remarks. See “remarks” section of Oxysarcodexia n. sp. 2. Female was classified by
Tibana & Mello (1985), according to the shape of the T6+7, as syntergite partially divided into
two plates. Description of morphological structures of larvae from 1st, 2
nd and 3
rd instars has
been provided by Lopes (1943).
Distribution. NEOTROPICAL. Brazil (Amapá, Amazonas, Goiás, Mato Grosso, Mato
Grosso do Sul, Minas Gerais, Pará, Rio de Janeiro, São Paulo), Colombia (Antioquia), Costa
Rica, Ecuador, Guyana, Panama, Peru, Roraima, Trinidad and Tobago (Trinidad), Venezuela.
Biology. In addition to human feces, attraction of adults of O. angrensis have been
reported by fish, mouse and rat carcasses, marine animals, rotting beef lung, chicken viscera,
and, at costal area, for crabs, rotten Syagrus comosa (Mart.) Mart. (Arecaceae), a species of a

42
palmtree, and rotten banana plus brown sugar (Lopes 1973b; Moretti et al. 2008; Sousa et al.
2011; Ramírez-Mora et al. 2012). No differences in attractiveness of this species by human
feces, chicken viscera and mouse carcasses baits were observed by Linhares (1981). Human
feces have seen being used as a natural rearing substrate (Lopes 1973b; D‟Almeida 1994).
Agar plus powder milk and another medium with a kind of jelly called “gelose” plus horse
blood serum and commercial yeast have been used successfully as artificial rearing sources for
the establishment and maintenance of colonies in laboratory. The developing from 1st instar to
adults was reached in 14–17 days using the first medium, whereas with the last medium
occurred an increase of the larval length in comparison to larvae reared in the same medium
without the yeast (Lopes 1973b). Meat has also been used successfully for rearing O.
angrensis (Lopes 1943). Adults have been collected, besides actively, with malaise and
Shannon traps (Lopes & Tibana 1991) and also associated to cadavers (Oliveira-Costa et al.
2001). Sunlight instead of shaded areas is also referred as a preference of this species
(Linhares 1981). Lopes (1973b) pointed out O. angrensis preference by high humidity based
on self-experience in flesh flies collection done in Brazil, in different periods. However,
Yepes-Gaurisas et al. (2013), collecting flies in a forest area of Antioquia, Colombia, observed
higher abundance of this species in dry season, with strong attraction for fish and also
considering this species asynanthropic.
Material examined. ♂♂: BRAZIL: São Paulo, Mogi Guaçu, Campininha, 18.IX.2011;
C. G. P. Lima, M. D. Grella, N. M. Jimenez / Oxysarcodexia VIII; Mogi Guaçu-SP;
18/11/2011 (L2B-DBA) // BRAZIL: São Paulo, Campinas, UNICAMP, 30.XI.2011; C. M.
Souza / Oxysarcodexia sp.; Campinas-SP; 30/11/2011; 12 (L2B-DBA).
Oxysarcodexia augusta Lopes, 1946
(Appendix 8-C)
Type locality. Brazil, Rio de Janeiro, Guanabara, Méier. Lopes, 1946b: 84.
Depository of type material. Unknown.
Diagnosis. Male. Postocular plate with golden microtomentum. Ocellar setae not well-
developed. Thorax with golden microtomentum, more intense at humeral region. Three well-
differentiated dorsocentral post-sutural setae; one smaller can be present among these others.
Apical scutellar seta present. Legs blackish. Abdomen grayish with silvery microtomentum.

43
T3 with 2 lateral marginal setae. T4 with 1 median marginal and two lateral marginal setae. T5
with silvery microtomentum only. ST5 with parallel cleft edges and with setosity and setae on
the apex of the arms. Cerci, in lateral view, straight with expanded apexes of oblique upwards
edges. Setae ventrally on the cerci (lateral view) present in full extension. Apical shape of the
cerci (last ⅓ portion of the cercus; posterior view) smaller than the middle part. Conformation
of the cerci in posterior view is divergent. In lateral view, presence of ventroapical concavity
on distiphallus with serrated margins; presence of lateroapical furrow; distiphallic apical shape
rounded; sinuous distiphallic dorsal shape. Presence of lateroapical expansions, in dorsal view,
on the distiphallus. Pregonite with expanded base narrowing smoothly until the apex, which
presents same color along the entire extension. Postgonite with expanded base and sudden
narrowing at apex. Vesica with symmetrical branches; terminal lobes well-developed,
filamentous at most tapering to apex, with sclerotized texture; presence of spines only on
ventral surface; and presence of a rounded median projection of the main vesica branch.
Remarks. Three dorsocentral post-sutural setae are seen in O. augusta, Oxysarcodexia
bikini Dodge, 1966, Oxysarcodexia corolla Dodge, 1965, Oxysarcodexia flavipes Lopes &
Tibana, 1987, Oxysarcodexia grandis Lopes, 1946, Oxysarcodexia jamesi Dodge, 1968, O.
nitida, O. notata, O. peruviana, Oxysarcodexia plebeja Lopes, 1946, Oxysarcodexia
terminalis (Hall, 1937), Oxysarcodexia varia (Walker, 1836), and O. vittata. Female was
classified by Tibana & Mello (1985), according to the shape of the T6+7, as syntergite
undivided.
Distribution. NEOTROPICAL. Argentina (Misiones), Brazil (Minas Gerais, Rio de
Janeiro, Roraima, Santa Catarina, São Paulo).
Biology. This species has already been reared under laboratory conditions (Lopes
1973b). However, no further information about its development could be found. Attractiveness
has been reported to human feces, chicken viscera, fish, mouse carcass, marine animals,
banana plus brown sugar and rotten Syagrus comosa (Mart.) Mart. (Arecaceae), a species of a
palmtree (at coastal area) (Lopes 1969; Lopes 1973b; Mendes & Linhares 1993). Malaise and
Shannon traps have been used to collect this species (Lopes & Tibana 1991), as well as active
collections. At Rio de Janeiro Zoological Garden, this fly had been collected using a wind
oriented trap (W.O.T.), baited with rotting liver (Oliveira et al. 2002).

44
Material examined. ♂: BRAZIL: Minas Gerais, Belo Horizonte, Est. Ecológica,
UFMG Campus; 2–22.VIII.1993; S. D. Gaiman / NRM-DIPT 0014231 (NRM).
Oxysarcodexia aura (Hall, 1937)
Sarcophaga aura Hall, 1937
(Appendix 9-A)
Type locality. Bolivia, northern Chiquitos. Hall, 1937: 372.
Depository of type material. DEI.
Diagnosis. Male. Postocular plate with silveryish microtomentum. Ocellar setae well-
developed. Thorax with golden microtomentum, more intense at humeral region. Dorsocentral
post-sutural setae with 2 well differentiated and 1–3 anterior setae smaller. Apical scutellar
seta absent. Legs brownish. Abdomen darkish brown with golden microtomentum. T3 with 3
lateral marginal setae. T4 with 1 (weak) median marginal and 2 lateral marginal setae. T5 with
golden microtomentum laterally. ST5 with V-shaped cleft edges and with setae along the
edges of the arms. Cerci, in lateral view, straight with pointed apexes of concave edges.
Presence of setae ventrally on the cerci (lateral view) only in basal half. Apical shape of the
cerci (last ⅓ portion of the cercus; posterior view) with the same size as the middle part.
Conformation of the cerci in posterior view is divergent. In lateral view, presence of
ventroapical concavity on distiphallus with smooth margins; distiphallic apical shape rounded;
and straight distiphallic dorsal shape. Pregonite with base and apex with same size and color.
Postgonite with expanded base narrowing smoothly until the apex. Vesica with symmetrical
branches; terminal lobes well-developed, with rounded shape and sclerotized texture; without
any spines.
Remarks. Initially it has been identified as Amesothyrsus chilensis, Enderlein, 1928,
revised as Sarcophaga sp. Engel, 1931 and then identified, by the proposition of a new name,
as Sarcophaga aura Hall, 1937. This species is easily recognized by the very peculiar cerci,
straight in lateral view and very slender and spaced in posterior view; by the distiphallus with
a singular conformation; and also by the vesica shape.
Distribution. NEOTROPICAL. Bolivia, Brazil (Distrito Federal, Mato Grosso, Mato
Grosso do Sul, Minas Gerais).

45
Biology. Species attracted to chicken viscera (Lopes 1980) and pig carcasses exposed
in areas of Brazilian Cerrado (“savanna-like” vegetation) (Barros et al. 2008; Rosa et al.
2011).
Material examined. ♂♂: Campinas, Est. De Goyaz, Borgmeier et Lopes 14-XII-35 /
Sarcophaga aura ♂, Hall, 8.5.7., Det. H. S. Lopes / NRM-DIPT 0014232 (NRM) // Brasil:
MG Pirapora 20–29.xii.1978 C. B. Carvalho col. / Hybopygia aura (Hall) Det. H. S. Lopes
(MNRJ) // O. aura Faz. Do Glória/10 [from Uberlândia, Minas Gerais state, Brazil] (L2B-
DBA); O. aura Faz. Do Glória/10 [from Uberlândia, Minas Gerais state, Brazil] (L2B-DBA).
Oxysarcodexia aurata (Macquart, 1851)
Sarcophaga aurata Macquart, 1851
Sarcophaga taitensis Schiner, 1868
Sarcophaga obtusifrons Thomson, 1869
Sarcophaga vesica Hall, 1933
Dasyproctia auricauda Enderlein, 1928
Oxysarcodexia lapitana Lehrer & Barbet, 2008 [not available name]
(Appendix 9-B)
Type locality. Oceania, probably Society Islands, Tahiti [“Océanie”]. Macquart, 1851:
207.
Depository of type material. MNHN.
Diagnosis. Male. Postocular plate with golden microtomentum. Ocellar setae well-
developed. Thorax and abdomen with silvery microtomentum. Dorsocentral post-sutural setae
with 2 well differentiated and 1–3 anterior setae smaller. Apical scutellar seta absent. Legs
blackish. T3 with 3 lateral marginal setae. T4 with 1 median marginal and 3 lateral marginal
setae. T5 with golden microtomentum in part. ST5 with parallel cleft edges and with setae on
the apex of the arms. Cerci, in lateral view, sinuous with normal apexes (same size as median
area) of concave edges. Setae ventrally on the cerci (lateral view) present at apical ⅓ portion.
Apical shape of the cerci (last ⅓ portion of the cercus; posterior view) with the same size as
the middle part. Conformation of the cerci in posterior view is divergent. In lateral view,
distiphallus ventroapically with smooth margins; distiphallic apical shape rounded and straight
distiphallic dorsal shape. Presence of lateral lobes on distiphallus and of a large distiphallic

46
dorsoapical swelling. Pregonite with base and apex with the same size and apex darker than
the base. Postgonite with expanded base narrowing smoothly until the apex. Vesica with
symmetrical branches; terminal lobes well-developed, filamentous at most tapering to apex,
with partially membranous texture; presence of spines only on ventral surface; and presence of
an angular median projection of the main vesica branch.
Remarks. Pape (2008), after revising nominal species of Sarcophagidae described by
Macquart from the Australasian/Oceanian region, proposed O. taitensis as a junior synonym of
O. aurata, although the first name have been widely used in several manuscripts.
Oxysarcodexia lapitana Lehrer & Barbet, 2008, a relatively recent name proposed, is indeed
O. aurata. In spite of material used for the proposition of the name O. lapitana has not seen,
the Lehrer & Barbet‟s illustration (Lehrer & Barbet 2008, fig. 1) provided very clear and
detailed elements enabling comparison between species and the statement of this synonym. It
is also important to stress O. lapitana wasn‟t correctly proposed, once all the International
Code of Zoological Nomenclature requirements weren‟t fulfil and, therefore, it is considered
not available. Oxysarcodexia aurata presents a large membranous dorsoapical swelling on
distiphallus as observed on Oxysarcodexia eberti Lopes & Tibana, 1987, Oxysarcodexia
galeata (Aldrich, 1916), Oxysarcodexia intona (Curran & Walley, 1934), Oxysarcodexia
peltata (Aldrich, 1916) and Oxysarcodexia varia (Walker, 1836). Cerci shape (lateral view)
and the large membranous dorsoapical swelling on the distiphallus are similar characters seen
in O. aurata and O. peltata. This species was included, besides Oxysarcodexia culminata
(Aldrich, 1916), Oxysarcodexia culmiforceps Dodge, 1966, Oxysarcodexia fringidea (Curran
& Walley, 1934), O. intona and O. peltata, in “peltata group”, e.g., possesses the genital
tergite of females reduced, membranous and with 2 spiracles, sternites 6+7 and 8 narrower and
the tergite 5 with lateral edges touching themselves ventrally (Lopes 1975c; Tibana & Mello
1983a). Female was classified by Tibana & Mello (1985), according to the shape of the T6+7,
as syntergite membranous.
Distribution. NEOTROPICAL. Chile (Cauquenes), Colombia (Antioquia), Costa
Rica, Ecuador, Galápagos Islands (Albermale, Charles, Chatham, Duncan, Indefatigable,
Narborough, San Salvador), Panama (Barro Colorado Island), Peru.
AUSTRALASIAN/OCEANIAN. Fiji, French Polynesia (Austral Islands, Marquesas Islands,
Society Islands, Tubuai Islands), Tahiti, Tonga, Western Samoa.

47
Biology. Attraction of this species is reported by chicken viscera and fish (Ramírez-
Mora et al. 2012; Yepes-Gaurisas et al. 2013). In Antioquia, Colombia, Yepes-Gaurisas et al.
(2013) observed a frequency increase of O. aurata with the rain and a close relation with
human settlements due to the high synanthropic index detected.
Material examined. ♂♂: NEW CALEDONIA: Province Nord. Poindimié, near the
coast, 24–28.xi.2001, Johanson, Pape & Viklund / NRM-DIPT 0014339 (NRM) // Society Is.,
Tahiti Punaaina, 4.1.1978, N. H. L. Krauss leg. (ZMUC).
Oxysarcodexia avuncula (Lopes, 1933)
Sarcophaga avuncula Lopes, 1933
Sarcophaga avunculus Blanchard (1935), incorrect subsequent spelling of avuncula
Lopes, 1933
(Appendix 9-C)
Type locality. Brasil, Rio de Janeiro, Rio de Janeiro, Manguinhos. Lopes, 1933: 156.
Depository of type material. MNRJ.
Diagnosis. Male. Postocular plate with silveryish microtomentum. Ocellar setae well-
developed. Thorax with pale golden microtomentum, more evident at humeral portion.
Dorsocentral post-sutural setae with 2 well differentiated and 1–3 anterior setae smaller.
Apical scutellar seta present. Legs blackish. Abdomen grayish with golden microtomentum
more intense at the lateral portion of T3 and full extension of T4. T3 with 3 lateral marginal
setae. T4 with 1 median marginal and 2 lateral marginal setae. T5 with silvery microtomentum
only. ST5 with parallel cleft edges and with setosity and setae on the apex of the arms. Cerci,
in lateral view, sinuous with pointed apexes of concave edges. Setae ventrally on the cerci
(lateral view) present at apical ⅓ portion. Apical shape of the cerci (last ⅓ portion of the
cercus; posterior view) with the same size as the middle part. Conformation of the cerci in
posterior view is divergent. In lateral view, presence of ventroapical concavity on distiphallus
with smooth margins; distiphallic apical shape rounded; straight distiphallic dorsal shape.
Pregonite with expanded base narrowing smoothly until the apex as well as postgonite, except
the color which is darker in the apex on the pregonite. Vesica with symmetrical branches;
terminal lobes well-developed, with rounded shape, partially membranous texture; presence of

48
spines only along the edges; and presence of an angular median projection of the main vesica
branch.
Remarks. See “remarks” section of O. admixta. A good and detailed male terminalia
comparison of the sympatric species not unusually misidentified, O. avuncula, O. confusa, O.
diana and O. parva, is given by Silva & Mello-Patiu (2008). Female was classified by Tibana
& Mello (1985), according to the shape of the T6+7, as syntergite undivided. Larvae are
characterized by the presence of sinuous ribbons of festoon on psedocephalon and
conspicuous teeth on maxillae (Leite & Lopes 1987; Lopes & Leite 1986; 1987).
Distribution. NEOTROPICAL. Argentina (Misiones), Bolivia, Brazil (Ceará, Distrito
Federal, Goiás, Mato Grosso, Mato Grosso do Sul, Minas Gerias, Rio de Janeiro, Santa
Catarina, São Paulo, Pernambuco), Colombia (Antioquia), Ecuador, Mexico (Oxaca),
Paraguay, Peru, Trinidad and Tobago (Trinidad).
Biology. It has been reared on cattle dung (Mendes & Linhares 2002). This species
also presents attraction for human feces, chicken viscera, fish, mouse and pig carcasses,
marine animals and banana plus brown sugar (Lopes 1973b; Lopes 1975a; Dias et al. 1984c;
Mendes & Linhares 1993; Ramírez-Mora et al. 2012; Yepes-Gaurisas et al. 2013). It has been
collected in urban (collection made by the authors) and forest environments (Vasconcelos &
Araújo 2012). This species was also associated to the gum of a Brazilian pioneer tree,
Terminalia argentea Mart & Zucc (Combretaceae), collecting or, sometimes, ingesting its
exudate, probably due to the high concentration of complex carbohydrates present in this
resource (Boff et al. 2008).
Material examined. ♂♂: BRAZIL: São Paulo, Campinas, Sousas, 13.IV.2011; C. M.
Souza, D. L. Brancoli, F. Rezende / O. avuncula (L2B-DBA) // BRAZIL: Minas Gerais,
Extrema, Serra do Lopo, Pedra das Flores, 27.II.2012; A. G. Savino, M. P. Nassu, M. D.
Grella / Oxysarcodexia B, Extrema-MG; 27/02/2012; 3 (L2B-DBA).
Oxysarcodexia bakeri (Aldrich, 1916)
Sarcophaga bakeri Aldrich, 1916
(Appendix 10-A)
Type locality. Cuba, Habana. Aldrich, 1916: 270.
Depository of type material. USNM.

49
Diagnosis. Male. Postocular plate with golden microtomentum. Ocellar setae well-
developed. Thorax with golden microtomentum. Dorsocentral post-sutural setae with 2 well
differentiated and 1–3 anterior setae smaller. Apical scutellar seta present. Legs blackish.
Abdomen grayish with golden microtomentum. T4 with 2 median marginal setae. T5 with
golden microtomentum. ST5 with parallel cleft edges and with setosity. Cerci, in lateral view,
straight with pointed apexes of oblique upwards edges. Setae ventrally on the cerci (lateral
view) present in full extension. Apical shape of the cerci (last ⅓ portion of the cercus; in
posterior view) with the same size as the middle part. Divergent conformation of the cerci in
posterior view. In lateral view, distiphallus with serrated margins ventroapically; distiphallic
apical shape conic; straight distiphallic dorsal shape and presence of ventroapical projections.
Presence of a remarkable lighter area dorsoapically on the distiphallus. Pregonite with
expanded base and sudden narrowing at apex, which is darker than the base. Postgonite with
expanded base narrowing smoothly until the apex. Vesica with symmetrical branches; terminal
lobes well-developed, filamentous at most tapering to apex, with sclerotized texture; presence
of spines only along the edges; and presence of a rounded median projection of the main
vesica branch.
Remarks. This species is considered similar to Oxysarcodexia modesta Lopes, 1946,
diverging from this by the presence of apical scutellar setae, setosity on syntergosternite 7+8
more abundant and peculiarities on the distiphallus (Lopes 1946b). Female was classified by
Tibana & Mello (1985), according to the shape of the T6+7, as syntergite partially divided into
two plates. This species has been intercepted in imported container with cargoes from
Americas in Shanghai Port, China (Deng et al. 2011).
Distribution. NEARTIC. Mexico (Baja California Sur, Sonora, Zacatecas), USA
(Texas). NEOTROPICAL. Bahamas (New Providence), Brazil (Bahia, Distrito Federal, Goiás,
Mato Grosso, Pernambuco, Roraima), Chile (Tarapacá), Colombia (Antioquia), Cuba,
Dominica, Ecuador, El Salvador, Guadalupe, Haiti, Jamaica, Mexico (Chiapas, Guerrero,
Sinaloa, Tabasco, Yucatán), Peru, Puerto Rico, Turks & Caicos Island. PALEARTIC. China
(Shangai).
Biology. This species has already been reared at laboratory, using agar plus powder
milk, developing from 1st instar to adults in 15–17 days (Lopes 1973b). Attractiveness has
been reported by human feces, fermented fruits (grapes), fish, chicken viscera, and liver

50
(Flores & Dale 1995; Ramírez-Mora et al. 2012). Oxysarcodexia bakeri was collected on a
residential complex of Valle de Aburrá, surround of Medellín, Colombia (Salazar-Ortega et al.
2012), pointing a possibly relationship of this species with human settlements, which was
corroborated by a high synanthropic index found by Yepes-Gaurisas et al. (2013) in a
collection of this fly at another urban area of Medellín.
Material examined. ♂♂: Oxysarcodexia bakeri sp 24 / 7CP2 [from Colombia] (CE-
TdeA) // Oxysarcodexia bakeri sp 20 / sp20 TdeA 846 [from Colombia] (CE-TdeA) // Cuba,
Ancón Nw de Viñales Pirnasdel Rio J. Holmsn 26.VIII.65 / Oxysarcodexia bakeri Aldr. B.
Rohdendorf det. 1970. VI / bakeri Cuba (MNRJ).
Oxysarcodexia berlai Lopes, 1975
(Appendix 10-B)
Type locality. Brazil, Pará, Belém, Utinga. Lopes 1975c: 473.
Depository of type material. MNRJ.
Diagnosis. Male. Postocular plate with pale golden microtomentum. Ocellar setae not
well-developed. Thorax with golden microtomentum, more intense at humeral region.
Dorsocentral post-sutural setae with 2 well differentiated and 1–3 anterior setae smaller.
Apical scutellar seta present. Legs brownish. Abdomen grayish with silvery microtomentum.
T3 with 1 lateral marginal seta. T4 with 1 median marginal and 2 lateral marginal setae. T5
with silvery microtomentum. ST5 with parallel cleft edges and with setosity and setae on the
apical half of the arms. Cerci, in lateral view, straight with expanded apexes of straight edges.
Setae ventrally on the cerci (lateral view) absent only in middle portion. Apical shape of the
cerci (last ⅓ portion of the cercus; in posterior view) smaller than the middle part.
Conformation of the cerci in posterior view is divergent. In lateral view, presence of
ventroapical concavity on distiphallus with serrated margins; distiphallic apical shape
rounded; sinuous distiphallic dorsal shape. In dorsal view, presence of lateroapical expansions
on the distiphallus. Pregonite with expanded base and sudden narrowing at apex, which
presents same color along the entire extension. Postgonite with expanded base narrowing
smoothly until the apex. Vesica with symmetrical branches; terminal lobes well-developed,
filamentous at most tapering to apex, with sclerotized texture; presence of spines only on
ventral surface; and presence of a rounded median projection of the main vesica branch.

51
Remarks. The main difference between O. berlai and Oxysarcodexia cuernavaca
Dodge, 1966 is the cerci conformation, parallel in first species and divergent in the second one
(Lopes 1975c) and genitalia shape (Dodge 1966). See “remarks” section of Oxysarcodexia n.
sp. 1 for further discussion.
Distribution. NEOTROPICAL. Brazil (Maranhão, Pará, Pernambuco), Colombia.
Biology. Unknown.
Material examined. ♂♂: Avispas, Madre de Dios, PERU, 10–20.IX.1962; L. Pena
400m / Oxysarc. Berlai; ♂; Lopes; Det. H. S. Lopes / NRM-DIPT 0014262 (NRM) // Igarapé
PARAQUEÚ Rosário, MARANHÃO BRASIL / Berla / 20/22-XI-70 / Paratype /
Oxysarcodexia berlai ♂ n.sp. Det. H. S. Lopes (MNRJ).
Oxysarcodexia bicolor Lopes, 1946
(Appendix 10-C)
Type locality. Brazil, Rio de Janeiro, Itatiaia. Lopes, 1946b: 127.
Depository of type material. MNRJ.
Diagnosis. Male. Postocular plate with golden microtomentum. Ocellar setae not well-
developed. Thorax with golden microtomentum, contrasting with the silveryish
microtomentum of the abdomen. Dorsocentral post-sutural setae with 2 well differentiated and
1–3 anterior setae smaller. Apical scutellar seta absent. Legs brownish. T3 with 1 lateral
marginal seta. T4 with 1 median marginal and 2 lateral marginal setae. T5 without golden
microtomentum. ST5 with parallel cleft edges and with setosity. Cerci, in lateral view, sinuous
with normal (i.e. same size as median area) apexes of oblique upwards edges. Setae ventrally
on the cerci (lateral view) absent only in middle portion. Apical shape of the cerci (last ⅓
portion of the cercus; posterior view) smaller than the middle part. Conformation of the cerci
in posterior view is parallel. Presence of a remarkable constriction at middle portion of the
cerci, in posterior view. In lateral view, distiphallus with smooth margins ventroapically;
distiphallic apical shape rounded and sinuous distiphallic dorsal shape. Presence of a large
dorsoapical swelling on the distiphallus, in lateral view. Lateroapical expansions on
distiphallus present, in dorsal view. Pregonite with same color along the entire extension and
expanded base narrowing smoothly until the apex. Postgonite with expanded base and sudden
narrowing at apex. Vesica with symmetrical branches; terminal lobes reduced, with rounded

52
shape and membranous texture; presence of spines on both ventral and dorsal surfaces; and
presence of a rounded median projection of the main vesica branch.
Remarks. The unusual cerci shape (in lateral view) draws the attention in this species.
Female was classified by Tibana & Mello (1985), according to the shape of the T6+7, as
syntergite undivided.
Distribution. NEOTROPICAL. Argentina (Buenos Aires), Brazil (Rio de Janeiro, São
Paulo).
Biology. This species has already been reared on laboratory (Lopes 1973b), although
no further information about its development is available on the literature. It is attracted by
human and dog feces and rotten cow liver (Lopes 1973b; Mulieri et al. 2008; Mulieri et al.
2010; Mulieri et al. 2011). This fly has been collected in woodland of Buenos Aires coastline,
Argentina (Mulieri et al. 2008) and in suburban and rural areas of Almirante Brown district,
near to Buenos Aires, pointing a possibly level of asynanthropy. The occurrence of this
species was higher during warmer months (Mulieri et al. 2011). Oxysarcodexia bicolor is also
considered a flower visitor of Scutia buxifolia Reissek, shrubs of Rhamnaceae family (Mulieri
et al. 2010).
Material examined. ♂♂: Alto da Mosela, Petrópolis, 1200m, E. do Rio, Brasil / H. S.
Lopes, 20.XII.70 / NMR-DIPT 0014265 (NRM) // Cult 878 / Petrópolis, Tq. E. do Rio, Brasil
H. S. Lopes 5/♀ / Oxysarcodexia bicolor Lopes Det. H. S. Lopes (MNRJ) // Campos do
Jordão / Est. São Paulo [Brasil] 1600m 3-1945 Wygodzinsky leg. / Oxysarcodexia bicolor
Lop. Det. H. S. Lopes (MNRJ).
Oxysarcodexia bikini Dodge, 1966
(Appendix 11-A)
Type locality. Chile. Dodge, 1966: 685.
Depository of type material. UCC.
Diagnosis. Male. Postocular plate with silvery microtomentum. Ocellar setae not well-
developed. Thorax and abdomen with silvery microtomentum. Three well-differentiated
dorsocentral post-sutural setae; one smaller can be present among them. Apical scutellar seta
present. Legs blackish. T3 with 2 lateral marginal seta. T4 with 1 median marginal and 3
lateral marginal setae. ST5 reddish yellow with short arms. Cerci straight with expanded

53
apexes of oblique upwards edges, in lateral view. Setae ventrally on the cerci (lateral view)
absent only in middle portion. Apical shape of the cerci (last ⅓ portion of the cercus; in
posterior view) smaller than the middle part. Conformation of the cerci is parallel in posterior
view. Presence of a remarkable constriction at middle portion of the cerci, also in posterior
view. In lateral view, distiphallus with ventroapical concavity with serrated margins;
distiphallic apical shape rounded and sinuous distiphallic dorsal shape. Pregonite and
postgonite with expanded base narrowing smoothly until the apex and same color along the
entire extension. Vesica symmetric; terminal lobes reduced, with rounded shape, sclerotized
texture and presence of spines. This diagnosis is based on original description given by Dodge
(1966), on diagnosis provided by Lopes (1973a), and on the examination of one specimen (as
specified below) presenting some conservation issues (“oily” aspect compromising original
microtomentum and color, T5 partially broke and terminalia on glycerin lacking ST5).
Remarks. This species was included by Lopes (1973a) in “paulistanensis group” due
to the similarities, as anterior view of the vesica and distiphallus apex, shared with
Oxysarcodexia injuncta (Walker, 1857), Oxysarcodexia paulistanensis Prado & Fonseca, 1932
and Oxysarcodexia riograndensis Lopes, 1946 species. Features as last genital sternite small
and enclosed in the hind margin of ST8, genital tergite entire and a broad membranous region
between the genital and anal segments are ascribed for O. bikini female (Dodge 1966; Lopes
1973a). See also “remarks” section on O. augusta for further discussion.
Distribution. NEOTROPICAL. Chile (La Araucanía).
Biology. Unknown.
Material examined. ♂: El Camelo, Santiago, CHILE 29-XI-1954 L. E. Peña /
Oxysarc. bikini ♂ Dodge Det. H. S. Lopes (MNRJ).
Oxysarcodexia carvalhoi Lopes, 1946
(Appendix 11-B)
Type locality. Brazil, Minas Gerais, Cordisburgo. Lopes, 1946b: 92.
Depository of type material. MNRJ.
Diagnosis. Male. Postocular plate with golden microtomentum. Ocellar setae not well-
developed. Thorax with golden microtomentum, more intense laterally at humeral region, as
well as the abdomen, in which is more evident laterally on T4, and on entire extension of T5.

54
Dorsocentral post-sutural setae with 2 well differentiated and 1–3 anterior setae smaller.
Apical scutellar seta present. Legs blackish. T3 with 2 lateral marginal seta. T4 with 1 median
marginal and 2 lateral marginal setae. T5 with golden microtomentum. ST5 with parallel cleft
edges and with setosity. Cerci, in lateral view, sinuous with expanded apexes of oblique
upwards edges. Setae ventrally on the cerci (lateral view) present on apical ⅓ portion. Apical
shape of the cerci (last ⅓ portion of the cercus; in posterior view) smaller than the middle part.
Conformation of the cerci in posterior view is divergent. In lateral view, distiphallus with
smooth margins ventroapically, presence of a lateroapical furrow and ventroapical projections.
Distiphallic anteroapical shape strongly concave, apical shape conic and straight distiphallic
dorsal shape. Pregonite and postgonite with same color along the entire extension and
expanded base narrowing smoothly until the apex. Vesica with symmetrical branches; terminal
lobes reduced, filamentous at most tapering to apex and sclerotized texture; presence of lateral
lobes (i.e. division of the vesica coming from or close to the basal branch of the vesica, placed
laterally to phallic tube) and spines only ventral surface; and presence of a rounded median
projection of the main vesica branch.
Remarks. See O. admixta “remarks” section. Female was classified by Tibana &
Mello (1985), according to the shape of the T6+7, as syntergite undivided.
Distribution. NEOTROPICAL. Brazil (Amapá, Ceará, Mato Grosso, Minas Gerais,
Pará, Rio de Janeiro, São Paulo), Ecuador, Guyana.
Biology. This fly has been collected using different baits as human feces, chicken
viscera, mouse and rat carcasses, fish, banana and brown sugar (Lopes 1975a; Dias et al.
1984c; Moretti et al. 2008). Human feces, mouse and rat carcasses were the substrates
reported being used for rearing this species under natural conditions (Lopes 1973b; Moretti et
al. 2008). At laboratory, O. carvalhoi has been reared on agar plus powder milk, developing
from 1st instar to adults in 14–17 days (Lopes 1973b).
Material examined. ♂♂: BRAZIL: São Paulo, Campinas, UNICAMP, 05.XII.2011;
C. M. Souza / Oxysarcodexia sp. 1; Campinas-SP; 05/12/2011; 15 (L2B-DBA) // BRAZIL:
São Paulo, Campinas, UNICAMP, 05.XII.2011; C. M. Souza / Oxysarcodexia sp. 1;
Campinas-SP; 05/12/2011; 15 (L2B-DBA).
Oxysarcodexia chaetopygialis (Williston, 1896)

55
Sarcophaga chaetopygialis Williston, 1896
(Appendix 11-C)
Type locality. Saint Vincent. Williston, 1896: 366.
Depository of type material. AMNH.
Diagnosis. Male. Postocular plate with golden microtomentum. Ocellar setae well-
developed. Thorax with grayish with pale golden microtomentum laterally. Dorsocentral post-
sutural setae with 2 well differentiated and 1–3 anterior setae smaller. Apical scutellar seta
absent. Legs brownish. Abdomen with silvery microtomentum. T3 with 2 lateral marginal
setae. T4 with undifferentiated median marginal and 1 lateral marginal seta. T5 without golden
microtomentum. ST5 with parallel cleft edges and with setosity and setae along the edges of
the arm. Cerci sinuous with pointed apexes of oblique upwards edges, in lateral view. Setae
ventrally on the cerci (lateral view) present on apical ⅓ portion. Apical shape of the cerci (last
⅓ portion of the cercus; in posterior view) same size as the middle part. Conformation of the
cerci in posterior view is parallel. In lateral view, distiphallus short and broad, with smooth
margins ventroapically; distiphallic apical shape square/oblong and sinuous distiphallic dorsal
shape. Pregonite broad, with same color along the entire extension (blackish) and base and
apex with same size, which can, depends on the genitalia exposure, hide the vesica. Postgonite
with expanded base narrowing smoothly until the apex. Vesica with symmetrical branches;
terminal lobes reduced, with rounded shape and membranous texture; without spines; but with
the presence of a rounded median projection of the main vesica branch.
Remarks. This species is recognized by the sinuosity of the cerci and the notable
distiphallus of light yellow color and enlarged ventroapically. Pregonite is dark brownish and
very broad, not unusually “hiding” the vesica. As already stressed by Dodge (1965), O.
chaetopygialis is the unique species of the genus presenting setulae on R1, even though a very
few (about 1–3 setulae).
Distribution. NEOTROPICAL. Jamaica (Saint Vincent).
Biology. Unknown.
Material examined. ♂♂: Majorca 1500‟ St. Vicent, W. I., VII–VIII.1972 / Malaise
trap, A. D. Harrison / Oxysarcodexia chaetopygialis (Will.) det. Lopes (ZMUC) // Majorca
1500‟ St. Vicent, W. I., VII–VIII.1972 / Malaise trap, A. D. Harrison / Oxysarcodexia
chaetopygialis ♂ (Will.) Det. Lopes (MNRJ).

56
Oxysarcodexia cingarus (Aldrich, 1916)
Sarcophaga cingarus Aldrich, 1916
Sarcophaga cingaris Hall (1929), incorrect subsequent spelling of cingarus Aldrich,
1916
(Appendix 12-A)
Type locality. Panama, Natrona. Aldrich, 1916: 288.
Depository of type material. USNM.
Diagnosis. Male. Postocular plate with pale golden microtomentum. Ocellar setae not
well-developed. Thorax with pale golden microtomentum more evident laterally, same for the
abdomen. Dorsocentral post-sutural setae with 2 well differentiated and 1–3 anterior setae
smaller. Apical scutellar seta absent. Legs brownish. T3 with 3 lateral marginal setae. T4 with
1 median marginal and 2 lateral marginal setae. T5 with golden microtomentum only laterally.
ST5 with parallel cleft edges and with setosity and setae on the apex of arms. Cerci, in lateral
view, straight with pointed apexes of oblique upwards edges. Setae ventrally on the cerci
(lateral view) present on apical ⅓ portion. Apical shape of the cerci (last ⅓ portion of the
cercus; in posterior view) smaller than the middle part. Conformation of the cerci in posterior
view is parallel. Presence of a remarkable constriction at middle portion of the cerci, also in
posterior view. In lateral view, distiphallus with ventroapical concavity of smooth margins;
distiphallic apical shape square/oblong and straight distiphallic dorsal shape. Pregonite with
same color along the entire extension and expanded base narrowing smoothly until the apex.
Postgonite with expanded base and sudden narrowing at apex. Vesica with symmetrical
branches; terminal lobes reduced, with filamentous at most tapering to apex shape and
sclerotized texture; without spines; and presence of an angular median projection of the main
vesica branch.
Remarks. Vesica of O. cingarus is one of the most simple shape and least ornamented
one among the species included in the genus. Phallus resemble somehow that seen in
Oxysarcodexia wygodzinsky Lopes & Tibana, 1987, although apex seems more angulated on
O. cingarus, and vesica and cerci shape are completely different for each species. Female was

57
classified by Tibana & Mello (1985), according to the shape of the T6+7, as syntergite
undivided.
Distribution. NEARTIC. Canada (Quebec, Ontario), USA (Arkansas, Colorado,
Florida, Iowa, Kansas, New Jersey, New York, Pennsylvania, South Carolina).
Biology. Adults of this fly has been considered scavengers according to their food
habitats, once they were collected associated to pig carcasses exposed on a tree as much as in
the water (Payne & King 1972). Predatism of O. cingarus by the wasp Oxybelus uniglumis
(Linnaeus, 1758) (Hymenoptera: Sphecidae) has been recorded in the literature (Peckham et
al. 1973). This fly was one of the species used to study the phylogeny of Sarcophagidae based
on a molecular approach (mtDNA fragments) (Stamper et al. 2012).
Material examined. ♂♂: Great Falls, Oct. 3, 15 Va / W. L. McAfee, Collector /
Sarcophaga cingarus Ald., Det. J. B. Malloch (ZMUC) // La Fayette, La. Det. 9-11-1947
Follow by trap USPHN: LaF 2 / Oxysarcodexia cingarus Ald. Det. E. R. Dodge 1957
(MNRJ).
Oxysarcodexia comparilis (Reinhard, 1939)
Sarcophaga comparilis Reinhard, 1939
Oxysarcodexia omissa Lopes, 1946
(Appendix 12-B)
Type locality. USA, Texas, Donna. Reinhard, 1939: 66.
Depository of type material. Unknwon.
Diagnosis. Male. Postocular plate with pale golden microtomentum. Ocellar setae not
well-developed. Thorax and abdomen with pale golden microtomentum. Dorsocentral post-
sutural setae with 2 well differentiated and 3 anterior setae smaller. Apical scutellar seta
absent. Legs blackish. T3 with 3 lateral marginal setae. T4 with 1 median marginal and 3
lateral marginal setae. T5 with golden microtomentum along the entire extension. ST5 with
parallel cleft edges and with setosity and setae on the apex of arms. Cerci straight with normal
apexes (i.e. same size as median area) of oblique upwards edges, in lateral view. Setae
ventrally on the cerci (lateral view) present on apical half portion. Apical shape of the cerci
(last ⅓ portion of the cercus; posterior view) smaller than the middle part. Conformation of the
cerci in posterior view is parallel. In lateral view, distiphallus with smooth margins

58
ventroapically; distiphallic apical shape rounded and sinuous distiphallic dorsal shape.
Pregonite with expanded base narrowing smoothly until the apex, which is darker than the
base. Postgonite broad with expanded base and sudden narrowing at apex same color along the
entire extension (darker than pregonite). Vesica with symmetrical branches; terminal lobes
reduced, with filamentous at most tapering to apex shape and sclerotized texture; without
presence of spines; and presence of a rounded median projection of the main vesica branch.
Remarks. This species is considered similar to Oxysarcodexia confusa Lopes, 1946
and Oxysarcodexia parva Lopes, 1946, which the most evident difference is seen on cerci
apex (Lopes 1975c). Reinhard pointed out similarities of O. comparilis with O. culminata,
stating characteristics of genitalia and the lighter parafacialia and parafrontalia
microtomentum as differences between them (Lopes 1946b). Terminalia of O. comparilis
resemble Oxysarcodexia occulta Lopes, 1946, although vesica of O. comparilis present
terminal lobes laterolaterally directed and characteristics as an angular median projection of
the main vesica branch, elongation of basal area of terminal lobes, bigger vesica size with
terminal lobes ventroapically directed, surstylus conic and cerci sinuous are seen only in the
last species.
Distribution. NEARTIC. Mexico (Districto Federal, Morelos), USA (Texas).
NEOTROPICAL. Ecuador, El Salvador, Mexico (Jalisco, Veracruz).
Biology. Unknown. Information available is only about the type of collection, active,
using an entomological net (Lopes 1946b).
Material examined. ♂: Acatlipa Morelos July 29 1950 Coll. N. G. Dorns /
Oxysarcodexia comparilis ♂ (Reinh.) Det. H. S. Lopes (MNRJ).
Oxysarcodexia conclausa (Walker, 1861)
Sarcophaga conclausa Walker, 1861 [female]
Sarcophaga ochripyga Wulp, 1895
Sarcophaga australis Aldrich, 1916
Sarcophaga ochropyga Mattos (1919), incorrect subsequent spelling of ochripyga
Wulp, 1895
Sarcophaga ochrypyga Lopes (1933), incorrect subsequent spelling of ochripyga
Wulp, 1895

59
(Appendix 12-C)
Type locality. Mexico. Walker, 1861: 309.
Depository of type material. BMNH.
Diagnosis. Male. Postocular plate with pale golden microtomentum. Ocellar setae not
well-developed. Thorax grayish with pale golden microtomentum. Dorsocentral post-sutural
setae with 2 well differentiated and 1–3 anterior setae smaller. Apical scutellar seta absent.
Legs brownish. T3 with 1 lateral marginal seta. T4 with 1 median marginal and 2 lateral
marginal setae. T5 with golden microtomentum along the entire extension. ST5 with parallel
cleft edges and with setosity. Cerci, in lateral view, straight with expanded apexes of oblique
upwards edges. Setae ventrally on the cerci (lateral view) present on apical ⅓ portion. Apical
shape of the cerci (last ⅓ portion of the cercus; posterior view) with same size as the middle
part. Conformation of the cerci in posterior view is parallel. In lateral view, distiphallus with
serrated margins ventroapically; distiphallic apical shape rounded and straight distiphallic
dorsal shape. Presence of a dorsoapical swelling (“finger-like”) on the distiphallus, in lateral
view. Pregonite with expanded base and sudden narrowing at apex, which is darker than the
base. Postgonite with expanded base and sudden narrowing at apex. Vesica with symmetrical
branches; terminal lobes well-developed, with square shape and sclerotized texture; presence
of spines only along the edges; and presence of an angular median projection of the main
vesica branch.
Remarks. Dorsoapical swelling “finger-like” of distiphallus, distiphallus apical
conformation and vesica with oblong shape and serrated edges resemble to those
characteristics of Oxysarcodexia morretesi Tibana & Mello, 1983 and O. thornax, despite of
apical shape of the cerci differing among O. conclausa and these other species. See also
“remarks” section on O. afficta. Female was described by Lopes (1975b) and classified by
Tibana & Mello (1985), according to the shape of the T6+7, as syntergite partially divided into
two plates. Larvae are known and were described by Knipling (1936).
Distribution. NEARTIC. Canada (Quebec), Mexico (Baja California Sur, Guajanuto,
Morelos, San Luis Potosí), USA (Arizona, California, Illinois, Indiana, Louisiana, Missouri,
New York, Oklahoma, Texas). NEOTROPICAL. Chile (La Araucanía, Patagonia, Tarapacá),
Colombia (Antioquia), Costa Rica (Alajuela, Guanacaste, San José), Ecuador, El Salvador,
Guatemala, Honduras (Tegucilgalpa), Mexico (Chiapas, Guerrero, Jalisco, Nayarít, Sinaloa,

60
Tabasco, Veracruz, Yucatán), Panama, Peru, Trinidad and Tobago (Trinidad), Venezuela
(Aragua).
Biology. This species is close related to bovine dung (Blume 1985), and due to its
sazonality (more active with the onset of the first spring which coincides with the emergence
of Haematobia irritans (Linnaeus, 1758) (Diptera: Muscidae)) and for being rearing on cattle
dung, it was considering a possible candidate for the natural control of the horn fly (Kunz
1978). Strong necrophagous behavior has been indicated by Yepes-Gaurisas et al. (2013),
further a high synanthropic index, pointing out this species preference for human settlements.
Collections with entomological net and with traps for collecting fruit flies are mentioned by
Lopes (1946b). Attractiveness is marked by fish, liver, chicken viscera and pig carcass (Flores
& Dale 1995; Garcés et al. 2004; Ramírez-Mora et al 2012; Yepes-Gaurisas et al. 2013).
Oxysarcodexia conclausa is also considered a floral visitor of Bdallophyton bambusarum
Liebm. (Rafflesiaceae) (Gacía-Franco & Rico-Gray 1997).
Material examined. ♂♂: Hidalgo Co. Tx., 4.X.1986; dysentery fly trap; USPUS Mi /
Oxysarcodexia ochripyga (ZMUC) // COLOMBIA Palmira, Valle 1 Mar 1971 1006m 165 G.
P. Waldbauer / Oxysarcodexia ochripyga Wulp, H. S. Lopes det. (MNRJ).
Oxysarcodexia confusa Lopes, 1946
(Appendix 13-A)
Type locality. BRASIL, RIO DE JANEIRO, Miguel Pereira. Lopes, 1946b:96.
Depository of type material. MNRJ.
Diagnosis. Male. Postocular plate with golden microtomentum. Ocellar setae well-
developed. Thorax with golden microtomentum, contrasting with the silveryish
microtomentum of the abdomen. Dorsocentral post-sutural setae with 2 well differentiated and
1–3 anterior setae smaller. Apical scutellar seta absent. Legs brownish. T3 with 2 lateral
marginal seta. T4 with 1 median marginal and three lateral marginal setae. T5 normally
without golden microtomentum, in a few cases a pale golden microtomentum can be present
laterally. ST5 with parallel cleft edges and with setosity. Cerci, in lateral view, straight with
expanded apexes of oblique upwards edges. Setae ventrally on the cerci (lateral view) absent
only in middle portion. Apical shape of the cerci (last ⅓ portion of the cercus; in posterior
view) same size as the middle part. Conformation of the cerci in posterior view is parallel.

61
Presence of a remarkable constriction at middle portion of the cerci, also in posterior view. In
lateral view, distiphallus with ventroapical concavity of smooth margins; distiphallic apical
shape rounded and straight distiphallic dorsal shape. Pregonite and postgonite with same color
along the entire extension and expanded base narrowing smoothly until the apex. Vesica with
symmetrical branches; terminal lobes reduced, with rounded shape and partially membranous
texture; presence of spines on both ventral and dorsal surfaces; and presence of a rounded
median projection of the main vesica branch.
Remarks. A good and detailed male terminalia comparison of the sympatric species
not unusually misidentified, O. avuncula, O. confusa, O. diana and O. parva, is given by Silva
& Mello-Patiu (2008). Distiphallus of O. confusa, in lateral view, is very similar to
Oxysarcodexia molitor (Curran & Walley, 1934), differing by the internal plates (spinous on
O. molitor) and the composition of the glans (Lopes 1975c). Female was classified by Tibana
& Mello (1985), according to the shape of the T6+7, as syntergite undivided. Larvae are
characterized by the presence of sinuous ribbons of festoon on psedocephalon and
conspicuous teeth on maxillae, same as O. thornax, differing from it by the more scattered
teeth of the maxillae and by the festoon arms (Leite & Lopes 1987; Lopes & Leite 1986;
1987). See also “remarks” section on O. comparilis for further discussion.
Distribution. NEOTROPICAL. Argentina (Misiones), Brazil (Mato Grosso, Mato
Grosso do Sul, Minas Gerais, Paraná, Rio de Janeiro, Santa Catarina, São Paulo).
Biology. Attractiveness by human feces, chicken viscera, mouse and fish carcasses and
banana plus brown sugar is reported (Lopes 1973b; Dias et al. 1984c; Mendes & Linhares
1993). This fly has been found rearing on human feces, under natural conditions, and at
laboratory, it has been reared on agar plus powder milk for 24h, then transferred to meat to
complete the development (Lopes 1973b). Studying synanthropy of flesh flies from Curitiba,
Brazil, Ferreira (1979) collected O. confusa using a trap baited with fish, chicken liver and
human feces, observing this species wasn‟t often associated with inhabited areas.
Material examined. ♂♂: Petrópolis, Tq; E. do Rio, Brasil, H. S. Lopes, 2.69 /
Oxysarc. confusa, Lop., ♂, Det. H. S. Lopes / NRM-DIPT 0014285 (NRM) // IGUASSÚ,
Paraná XII-941, Com. E. N. V. / NRM-DIPT 0014287 (NRM) // Taquara, Petrópolis, E. do
Rio, Brasil / H. S. Lopes, 16.II.75 / Oxysarcodexia confusa, Lopes. Det. H. S. Lopes (ZMUC)

62
// S. Paulo – Cantareira Serra L. Trav. F. Q. 30.VIII.935 / Paratype / Oxysarcodexia confusa
sp.n. Lopes – det 1944 (MNRJ).
Oxysarcodexia corolla Dodge, 1965
(Appendix 13-B)
Type locality. Jamaica, Saint Andrews, Second Breakfast Spring. Dodge, 1965: 508.
Depository of type material. WSU.
Diagnosis. Male. Postocular plate yellowish gray. Ocellar setae weak. Thorax gray and
abdomen with silvery microtomentum. Dorsocentral post-sutural setae with 3 well
differentiated, a small seta can be present among them. Apical scutellar seta present, although
considered small or weak. Legs blackish. Abdomen grayish with pale golden microtomentum
laterally. T4 with long marginal setosity, but without setae. ST5 with a deep cleft. Pregonite
with darker apex, stouter and curved. Postgonite is slender and nearly straight. Cerci brownish
red with black apexes, not divergent apically and a conspicuous preapical “tooth”. Distiphallus
straight with compressed apex and an anterior elongation pointed inward, and bearing a
preapical flap around dorsal and lateral sides. Vesica is black, broad, with an asymmetric
finger-shaped process on the left side. This is a synopsis of the original description (Dodge
1965) and of a redescription (Lopes 1978).
Remarks. See “remarks” section on O. augusta. Female was classified by Tibana &
Mello (1985), according to the shape of the T6+7, as syntergite undivided.
Distribution. NEOTROPICAL. Jamaica (St. Andrews).
Biology. Unknown.
Oxysarcodexia cuernavaca Dodge, 1966
(Appendix 13-C)
Type locality. Mexico, Morelos, Cuernavaca. Dodge, 1966: 685.
Depository of type material. USNM.
Diagnosis. Male. Postocular plate with silvery microtomentum. Weak ocellar setae.
Thorax gray, slightly yellow laterally. Dorsocentral post-sutural setae with 2 well
differentiated and 2 anterior setae smaller. Apical scutellar seta is absent. Legs blackish.
Abdomen black and brown apically, with silvery microtomentum. T3 with median marginal

63
seta. T4 yellow with a marginal row of setae. T5 with golden microtomentum. Cerci yellow
with divergent apexes (posterior view), and straight shape and a strong preapical “tooth”, in
lateral view. Pregonite long and curved. Postgonite without seta. Large vesica with flattened
and spined lobes, presenting broad and thin margins. This is a synopsis of original description
given by Dodge (1966).
Remarks. It is considered similar to O. ventricosa, by the similar cerci with divergent
apexes (Dodge 1966). See also “remarks” section of O. berlai for further discussion.
Distribution. NEARTIC. Mexico (Jalisco, Morelos, San Luis Potosí).
Biology. Unknown.
Oxysarcodexia culmiforceps Dodge, 1966
Oxysarcodexia culminiforceps Lopes (1969), incorrect subsequent spelling of
culmiforceps Dodge, 1966
(Appendix 14-A)
Type locality. Brazil, Rio de Janeiro, Rio de Janeiro. Dodge, 1966: 687.
Depository of lectotype species. MNRJ.
Diagnosis. Male. Postocular plate with golden microtomentum. Ocellar setae well-
developed. Thorax with golden microtomentum laterally. Dorsocentral post-sutural setae with
2 well differentiated and 1–3 anterior setae smaller. Apical scutellar seta absent. Legs
blackish. Abdomen with silvery microtomentum. T3 with 2 lateral marginal setae. T4 with 1
median marginal and 2 lateral marginal setae. T5 with golden microtomentum laterally
(“stain-like”). ST5 with parallel cleft edges and with setosity and setae along the edges of the
arms. Cerci, in lateral view, bent backwards (very conspicuous) with pointed apexes of
oblique upwards edges. Setae ventrally on the cerci (lateral view) present on apical ⅓ portion.
Apical shape of the cerci (last ⅓ portion of the cercus; in posterior view) with same size as the
middle part. Conformation of the cerci in posterior view is divergent. Presence of a remarkable
constriction at middle portion of the cerci, also in posterior view. In lateral view, distiphallus
with a ventroapical concavity of serrated margins ventroapically; presence of a lateroapical
furrow; distiphallic apical shape conic and sinuous distiphallic dorsal shape. Presence of a
large dorsoapical swelling on the distiphallus, in lateral view. Lateroapical expansions present
on distiphallus, in dorsal view. Pregonite with expanded base narrowing smoothly until the

64
apex, which is darker than the base. Postgonite with expanded base and sudden narrowing at
apex. Vesica with symmetrical branches; terminal lobes reduced, with filamentous at most
tapering to apex shape and partially membranous texture; presence of spines only along the
edges; and presence of an angular median projection of the main vesica branch.
Remarks. Initially it was misidentified as O. culminata although morphological
variations, especially at male genitalia, were pointed out by Lopes (1946b), reason of it is
considered a very variable species. Dodge (1966) confirmed these differences as belonging to
another species, proposing the new name O. culmiforceps. Differentiation of O. culmiforceps
from O. culminta is based on distiphallus apex and vesica details (Lopes 1975c). See
“remarks” section on O. aurata for further discussion. Female was classified by Tibana &
Mello (1985), according to the shape of the T6+7, as syntergite membranous.
Distribution. NEOTROPICAL. Argentina (Buenos Aires, Entreríos, Misiones), Brazil
(Distrito Federal, Espírito Santo, Minas Gerais, Paraná, Rio de Janeiro, Rio Grande do Sul,
Santa Catarina, São Paulo), Paraguay.
Biology. This species is attracted by human and dog feces, chicken viscera, rotten
bovine liver, fish, mouse and pig carcasses and banana plus brown sugar (Ferreira 1979; Dias
et al. 1984c; Mendes & Linhares 1993; Carvalho & Linhares 2001; Leandro & D‟Almeida
2005; Mariluis et al. 2007; Mulieri et al. 2008; Mulieri et al. 2010; Mulieri et al. 2011; Vairo
et al. 2011). Despite of this variety of substrates, it has been observed no differences in
attractiveness among human feces, chicken viscera and mouse carcasses (Linhares 1981; Dias
et al. 1984c), besides no preference for sunlight or shaded areas (Linhares 1981). However,
Ferreira (1979) recorded this species preference by human feces, linking this fact to a possible
negative relationship to the preference for inhabited areas. Grassland, woodland (Mulieri et al.
2008), urban, suburban and rural areas (Mulieri et al. 2011), forest, rural and urban areas (in
order of abundance in each site) at Brazilian South (Ferreira 1979), a Brazilian zoological
garden (Oliveira et al. 2002) and a forest area (our own data) are the environments where O.
culmiforeceps has already been collected on. This fly is also recognized as flower visitor of
Sebastiania brasiliensis Spreng. (Euphorbiaceae) and of Scutia buxifolia Reissek
(Rhamnaceae) (Mulieri et al. 2010).

65
Material examined. ♂: BRAZIL: Minas Gerais, Extrema, Serra do Lopo, Pedra das
Flores, 27.II.2012; A. G. Savino, M. P. Nassu, M. D. Grella / O. culmiforceps, Extrema-MG;
27/02/2012 (L2B-DBA).
Oxysarcodexia culminata (Aldrich, 1916)
Sarcophaga culminata Aldrich, 1916
(Appendix 14-B)
Type locality. Puerto Rico, Mayaguez. Aldrich, 1916: 289.
Depository of type material. AMNH.
Diagnosis. Male. Postocular plate with pale golden microtomentum. Ocellar setae
well-developed. Thorax grayish with golden microtomentum at humeral portion. Dorsocentral
post-sutural setae with 2 well differentiated and 1–3 anterior setae smaller. Apical scutellar
seta absent. Legs blackish. T3 with 2 lateral marginal setae. T4 with 1 median marginal and 3
lateral marginal setae. T5 with exclusively silvery microtomentum. ST5 with parallel cleft
edges and with setosity. Cerci, in lateral view, bent backwards with pointed apexes of oblique
upwards edges. Setae ventrally on the cerci (lateral view) present in apical ⅓ portion. Apical
shape of the cerci (last ⅓ portion of the cercus; in posterior view) with the same size as the
middle part. Conformation of the cerci in posterior view is parallel. Presence of a remarkable
constriction at middle portion of the cerci, in posterior view. In lateral view, distiphallus with
ventroapical concavity with serrated margins ventroapically; distiphallic apical shape conic
and sinuous distiphallic dorsal shape. Pregonite with expanded base narrowing smoothly until
the apex, which is darker than the base, as well as the postgonite, except for the color which is
the same along the entire extension. Vesica with symmetrical branches; terminal lobes
reduced, with rounded shape and partially membranous texture; presence of spines only on
ventral surface; and presence of an angular median projection of the main vesica branch.
Remarks. See “remarks” section on O. aurata, O. comparilis and O. culmiforceps.
Distribution. NEOTROPICAL. Argentina (Misiones), Brazil (Rio de Janeiro), Puerto
Rico.
Biology. This species has already been reared under laboratory conditions and the
attraction by human feces, rotten Syagrus comosa (Mart.) Mart. (Arecaceae), a species of a
palmtree, and rotten banana plus brown sugar (the last two at coastal area) has been reported

66
on the literature (Lopes 1973b). This author also noted the distribution of O. culminta in
highlands at Rio de Janeiro, Brazil.
Material examined. ♂: PUERTO RICO: Jayuya Bosque Estatal del Toro Negro,
0,7km SE, Cerro de Punta, Cordillera Central / 18-10-09N, 66-35-16W, 1195m, 9 June 1966,
J. Rawlins. C. Young, R. Davidson, W. Zanol, S. Thompson, M. Klinger / Carnegie Museum,
Specimen Number CMNH-67351 / NRM-DIPT 0014285 (NRM).
Oxysarcodexia cyanea Lopes, 1975
(Appendix 14-C)
Type locality. Dominica, Traglafar Falls. Lopes, 1975b: 475.
Depository of type material. NMNH.
Diagnosis. Male. Back of head gray. Ocellar setae well-developed. Thorax gray,
dorsocentral post-sutural setae with 2 well differentiated and 2 anterior smaller setae. Apical
scutellar seta is absent. Legs blackish. Abdomen grayish with silvery microtomentum. T4
without median marginal setae. ST5 reddish brown. Cerci yellow, slightly curved on apexes,
which are blackish. Parallel conformation of cerci and enlarged cerci apexes in posterior view.
Pregonite with expanded apex. Distiphallus sinuous on apex and with a small membranous
area dorsally. Vesica large with apical lobes bearing a few spines. This is a synopsis of the
original description given by Lopes (1975b).
Remarks. Distiphallus similar to Oxysarcodexia zayasi Dodge, 1956, although
differences are notably on cerci which is near to O. orbitalis, but differing from O. cyanea by
the distinct distiphallus. It is also distinguished from O. aurata due to the absence of a
dorsoapical swelling (called by Lopes as a “large membrane on penis apex”) (Lopes 1975b).
Female was classified by Tibana & Mello (1985), according to the shape of the T6+7, as
syntergite undivided.
Distribution. NEOTROPICAL. Dominica.
Biology. Unknown. There is only a mention in the original description paper about
some specimens collected on human feces, however without mention which species they refer
to (Lopes 1975b).
Oxysarcodexia cyaniforceps (Hall, 1933)

67
Sarcophaga cyaniforceps Hall, 1933
(Appendix 15-A)
Type locality. Panama, Barro Colorado Island. Hall, 1933: 282.
Depository of type material. AMNH.
Diagnosis. Male. Postocular plate golden microtomentum. Thorax gray and with
golden microtomentum on humeral region and laterally. Dorsocentral post-sutural setae with 2
well differentiated and 3 anterior smaller setae. Apical scutellar seta is absent. Abdomen
grayish with pale golden microtomentum laterally on T3 and on entire tergites T4 and T5. T4
with median marginal setae. T5 completely black. ST5 reddish with a deep cleft. Cerci with
sinuous shape (lateral view), enlarged and curved apexes. Distiphallus mostly membranous,
but with a pair of spinous plates internally. Large vesica with 2 pair of spinous lobes. This is a
synopsis of diagnosis given by Lopes (1946b; 1975c).
Remarks. Accordingly to drawings presented by Lopes (1975c), cerci seem similar to
the one presented by O. chaetopygialis and vesica resembles Oxysarcodexia floricola Lopes,
1975, although terminal lobes are ventroapically directed and basal portion is placed slightly
below distiphallus apex. See also “remarks” section of O. adunca for further discussion.
Distribution. NEOTROPICAL. Colombia (Antioquia), Costa Rica, Panama (Barro
Colorado Island, Corozal Canal Zone).
Biology. This fly is considered very rare and it was collected only one specimen in
urban area of Antioquia, Colombia, using chicken viscera as baits (Yepes-Gaurisas et al.
2013).
Oxysarcodexia diana (Lopes, 1933)
Sarcophaga diana Lopes, 1933
(Appendix 15-B)
Type locality. Brasil, Rio de Janeiro, Rio de Janeiro. Lopes, 1933:154.
Depository of type material. MNRJ.
Diagnosis. Male. Postocular plate with golden microtomentum. Ocellar setae not well-
developed. Thorax with golden microtomentum more intense at humeral portion. Dorsocentral
post-sutural setae with 2 well differentiated and 1–3 anterior setae smaller. Apical scutellar
seta absent. Legs brownish. Abdomen grayish with some shades of golden microtomentum

68
laterally. T3 with 3 lateral marginal setae. T4 with 1 median marginal and 1 lateral marginal
seta. T5 with golden microtomentum along the entire extension. ST5 with parallel cleft edges
and with setosity and setae on the apex of the arms. Cerci, in lateral view, sinuous with
expanded apexes of oblique upwards edges. Setae ventrally on the cerci (lateral view) present
in apical ⅓ portion. Apical shape of the cerci (last ⅓ portion of the cercus; posterior view)
smaller than the middle part. Conformation of the cerci in posterior view is parallel. In lateral
view, distiphallus with ventroapical concavity with smooth margins ventroapically;
distiphallic apical shape rounded and sinuous distiphallic dorsal shape. Pregonite with
expanded base narrowing smoothly until the apex, which is darker than the base. Postgonite
with expanded base and sudden narrowing at apex. Vesica with symmetrical branches;
terminal lobes well-developed, with rounded shape and membranous texture; presence of
spines only on ventral surface; and presence of a rounded median projection of the main
vesica branch.
Remarks. A good and detailed male terminalia comparison of the sympatric species
not unusually misidentified, O. avuncula, O. confusa, O. diana and O. parva, is given by Silva
& Mello-Patiu (2008). See O. admixta “remarks” section for further discussion. Female was
classified by Tibana & Mello (1985), according to the shape of the T6+7, as syntergite
undivided.
Distribution. NEARTIC. Mexico (Morelos, San Luis de Potosí). NEOTROPICAL.
Argentina (Misiones), Brazil (Distrito Federal, Ceará, Goiás, Mato Grosso, Mato Grosso do
Sul, Minas Gerais, Paraná, Rio de Janeiro, Roraima, Santa Catarina, São Paulo), Colombia
(Antioquia), Ecuador, El Salvador, Mexico (Chiapas), Paraguay, Trinidad and Tobago
(Trinidad).
Biology. This fly has already been reared on human feces (D‟Almeida 1989;
D‟Almeida 1994; Lopes 1973b; Lopes 1975c) – and it has great preference for this kind of
substrate for rearing (Mendes & Linhares 1993) –, on bovine feces of pasture (Marchiori
2000; Marchiori et al. 2001), cattle shed (Marchiori 2000), on fish (D‟Almeida 1986) and also
under laboratory conditions (Lopes 1973b). D‟Almeida (1984) noticed this species preference
for inhabited areas, collecting it with human feces, fish, bovine liver and banana plus brown
sugar as baits (in decreasing scale of abundance). Attractiveness has been reported by human
feces, fish, chicken viscera, bovine liver and lung, mouse and pig carcasses, crab, squid,

69
banana plus brown sugar and rotten Syagrus comosa (Mart.) Mart. (Arecaceae), a species of a
palmtree (at coastal area) (Lopes 1973b; Lopes 1975a; Linhares 1981; D‟Almeida 1984; Dias
et al. 1984c; Mendes & Linhares 1993; D‟Almeida & Lima 1994; Pamplona et al. 2000;
Carvalho & Linhares 2001; Leandro & D‟Almeida 2005; Barros et al. 2008; Barbosa et al.
2009; Ramírez-Mora et al. 2012; Yepes-Gaurisas et al. 2013). Analyzing the attractiveness of
three different baits, Linhares (1981) observed no differences for mouse carcass, human feces
and chicken viscera, although Mendes & Linhares (1993), in a study using the same baits,
pointed out O. diana preference for human feces. Sunlight instead of shaded areas is also
referred as a preference of this species (Linhares 1981). The higher frequency of occurrence of
adult females on chicken viscera and rodent carcasses baits suggest the use of these substrates
as proteic resources for the development of ovarian folicules (Mendes & Linhares 1993).
Besides actively, this fly has been collected using malaise Shannon and W.O.T. traps baited
with different substrates mentioned above (Lopes & Tibana 1991; Pamplona et al. 2000). At
Rio de Janeiro Zoological Garden, it was the third most abundant species among flesh flies
collected, showing a peak of occurrence in January and February (summer) and, consequently,
positive correlation with temperature and preference for urban areas and feces (available
abundantly at the zoo) (Oliveira et al. 2002).
Material examined. ♂♂: Oxysarcodexia diana sp 4 / TdeA 831 (CE-TdeA) [from
Colombia] // BRAZIL: Minas Gerais, Belo Horizonte, Est. Ecológica, UFMG, Campus, 2–
22.vii.1993, S. D. Gaiman / NRM-DIPT 0014598 (NRM) // GRAJAÚ Rio de Janeiro Lopes
10.XI.40 (MNRJ).
Oxysarcodexia eberti Lopes & Tibana, 1987
(Appendix 15-C)
Type locality. Brazil, Minas Gerais, Paracatu. Lopes & Tibana, 1987: 336.
Depository of type material. MNRJ.
Diagnosis. Male. Postocular plate with golden microtomentum. Ocellar setae not well-
developed. Thorax and abdomen with silvery microtomentum and slightly pale golden
microtomentum shades. Dorsocentral post-sutural setae with 2 well-differentiated and 3
anterior setae smaller. Apical scutellar seta absent. Legs blackish. T3 with 2 lateral marginal
setae. T4 without median marginal and with 2 lateral marginal setae. T5 with slightly pale

70
golden microtomentum, although silvery microtomentum more intense. ST5 with parallel cleft
edges and setosity. Cerci, in lateral view, bent backwards with expanded apexes of oblique
upwards edges. Setae ventrally on the cerci (lateral view) absent only in middle portion.
Apical shape of the cerci (last ⅓ portion of the cercus; in posterior view) smaller than the
middle part. Conformation of the cerci in posterior view is parallel. In lateral view,
distiphallus with smooth margins ventroapically; distiphallic apical shape square/oblong and
sinuous distiphallic dorsal shape. Presence of a large dorsoapical swelling on the distiphallus,
in lateral view. Lateroapical expansions on distiphallus, in dorsal view, present. Pregonite and
postgonite with expanded base and sudden narrowing at apex, which is darker than the base
only in pregonite. Vesica symmetric; terminal lobes well-developed, with square shape and
mostly sclerotized texture; presence of spines along the edges; and presence of an angular
median projection of the main vesica branch.
Remarks. See “remarks” section on O. aurata.
Distribution. NEOTROPICAL. Brazil (Distrito Federal, Mato Grosso, Mato Grosso
do Sul, Minas Gerais).
Biology. This species has been collected associated to pig carcass in areas of Brazilian
Cerrado (“savanna-like”) vegetation (Barros et al. 2008; Rosa et al. 2011).
Material examined. ♂: BRASIL: MT: Chap. Dos Guimarães Véu da Noiva – cerrado
S15°24‟ W5550‟ Shannon – peixe 16.i.2012 Mello-Patiu & Patiu col.
SISBIOTA/CNPq/FAPESP / Oxysarcodexia eberti Lopes & Tibana, 1987 Det. C. A. Mello-
Patiu (MNRJ).
Oxysarcodexia edwardsi Lopes, 1946
(Appendix 16-A)
Type locality. Colombia, Gorgona Island. Lopes, 1946b: 123.
Depository of type material. BMNH.
Diagnosis. Male. Postocular plate with pale golden microtomentum. Ocellar setae
well-developed. Thorax grayish and with pale golden microtomentum. Dorsocentral post-
sutural setae with 2 well differentiated and 3 anterior smaller setae. Apical scutellar seta is
absent. Legs blackish. Abdomen grayish with golden microtomentum laterally. T4 with one
pair of median marginal setae. T5 with golden microtomentum along the entire extension. ST5

71
with a deep cleft of V-shaped edges. Cerci reddish with blackish and “arrow-shaped” apexes.
Distiphallus reddish. Vesica of notably asymmetrical branches. This is a synopsis of the
original description given by Lopes (1946b).
Remarks. Comparison among drawings from O. edwardsi terminalia presented in the
original description (Lopes 1946b: Figs. 146–149) and other Oxysarcodexia species enables
recognize some similarities with O. parva and Oxysarcodexia trivialis (Wulp, 1895). Cerci is
very similar to that seen on O. parva, nevertheless vesica is completely different and more
similar to that of O. trivialis. In O. edwardsi the vesica presents an angular projection of the
main vesica branch, a more enlarged basal portion of terminal lobes and the apex of terminal
lobes ventroapical directed. For O. trivialis, the main vesica branch presents a rounded
projection, narrower basal portion of terminal lobes and the apex of terminal lobes dorsoapical
directed.
Distribution. NEOTROPICAL. Brazil (Rio de Janeiro), Colombia (Gorgona Island).
Biology. Collected using traps baited with cow liver and lung, fish and squid and
actively with feces (Pamplona et al. 2000).
Oxysarcodexia favorabilis (Lopes, 1935)
Sarcophaga favorabilis Lopes, 1935
(Appendix 16-B)
Type locality. Brazil, Rio de Janeiro, Angra dos Reis. Lopes, 1935: 318.
Depository of type material. MNRJ.
Diagnosis. Male. Postocular plate with golden microtomentum. Ocellar setae well-
developed. Thorax with golden microtomentum, contrasting with the silveryish
microtomentum of the abdomen. Three well differentiated dorsocentral post-sutural setae are
present. Apical scutellar seta absent. Legs dark brownish. T3 with 2 lateral marginal setae. T4
with 1 median marginal and 3 lateral marginal setae. T5 without golden microtomentum, only
silvery microtomentum is present. ST5 with parallel cleft edges and with setosity. Cerci, in
lateral view, bent backwards with pointed apexes of straight edges. Setae ventrally on the cerci
(lateral view) absent only in middle portion. Apical shape of the cerci (last ⅓ portion of the
cercus; in posterior view) smaller than the middle part. Conformation of the cerci in posterior
view is parallel. In lateral view, distiphallus with smooth margins ventroapically; distiphallic

72
apical shape square/oblong and sinuous distiphallic dorsal shape. In lateral view, presence of a
large dorsoapical swelling on the distiphallus, ventroapical projections and lateral lobes.
Lateroapical expansions on the distiphallus, in dorsal view, present. Pregonite with base and
apex with almost same size and same color along the entire extension. Postgonite with
expanded base and sudden narrowing at apex and same color along the entire extension.
Vesica symmetric; terminal lobes well-developed, with filamentous at most tapering to apex
shape, membranous texture and presence of spines.
Remarks. See “remarks” section on Oxysarcodexia 4 n. sp.
Distribution. NEOTROPICAL. Brazil (Rio de Janeiro).
Biology. Unknown.
Material examined. ♂: Angra dos Reis, Est. do Rio, Dario Mendes 8-934 / Holotype /
Typus / Sarcophaga favorabilis Lopes H. S. Lopes DET. – 935 / MNRJ/2307 (MNRJ).
Oxysarcodexia festiva Lopes & Tibana, 1987
(Appendix 16-C)
Type locality. Costa Rica, Cartago, Turrialba. Lopes & Tibana, 1987, 338.
Depository of type material. CAS.
Diagnosis. Male. Postocular plate with golden microtomentum. Ocellar setae with
about the size of smaller frontal setae. Thorax with golden microtomentum dorsally and on
mesonotum. Dorsocentral post-sutural setae with 2 well differentiated and 3 anterior smaller
setae. Apical scutellar seta is absent. Legs brown. Abdomen with yellowish gray
microtomentum. T4 with median marginal setae. ST5 with slender and elongated apical arms
covered with setosity on apexes. Cerci with pre-apical dorsal recurrent lobe and remarkable
setosity on apexes. Distiphallus curved forwards, with setosity anteroapically. Robust vesica
with setosity ventrally; apical lobes elongated, membranous and with a pair of spines. This is a
synopsis of the original description given by Lopes & Tibana (1987).
Remarks. Species easily distinguished from others by the peculiar cerci (Lopes &
Tibana 1987).
Distribution. NEOTROPICAL. Costa Rica.
Biology. Unknown.

73
Oxysarcodexia flavipes Lopes & Tibana, 1987
(Appendix 17-A)
Type locality. Argentina, Tucumán, Cerro, San Javier. Lopes & Tibana, 1987: 355.
Depository of type material. MNRJ.
Diagnosis. Male. Postocular plate with silvery microtomentum. Ocellar setae well-
developed. Thorax with pale golden microtomentum. Three well-differentiated dorsocentral
post-sutural setae. Apical scutellar seta absent. Legs yellowish. Abdomen with silveryish
microtomentum. T3 with one lateral marginal seta. T4 with 1 median marginal and 1 lateral
marginal seta. T5 without golden microtomentum, only yellowish on ventral edges. ST5 with
parallel cleft edges and with setosity and setae along the edges of the arms. Cerci, in lateral
view, straight with normal (i.e. same size as median area) apexes of oblique upwards edges.
Setae ventrally on the cerci (lateral view) present in apical ⅓ portion. Apical shape of the cerci
(last ⅓ portion of the cercus; in posterior view) smaller than the middle part. Conformation of
the cerci in posterior view is divergent. In lateral view, distiphallus with smooth margins
ventroapically; distiphallic apical shape conic and sinuous distiphallic dorsal shape. Presence
of a small dorsoapical swelling on the distiphallus, in lateral view. Lateroapical expansions on
distiphallus, in dorsal view, present. Pregonite with base and apex with the same size and same
color along the entire extension. Postgonite with expanded base and sudden narrowing at apex
and same color along the entire extension. Vesica symmetric; with presence of lateral lobes
(i.e. division of the vesica coming from or close to the basal branch of the vesica, placed
laterally to phallic tube); terminal lobes reduced, with rounded shape, sclerotized texture and
presence of spines on both ventral and dorsal surfaces.
Remarks. This species is distinctly recognized by the yellow legs, 3 dorsocentral post-
sutural setae, apical distiphallic apex and conspicuous vesica (Lopes & Tibana 1987). See also
“remarks” section on O. augusta.
Distribution. NEOTROPICAL. Argentina (Tucumán).
Biology. Unknown.
Material examined. ♂: Cerro San Javier Tucuman, Arg. 8.X.53 800m P.
Wygodzinsky / Holótipo / Oxysarcodexia flavipes n.sp. holotypus ♂ Det. H. S. Lopes & R.
Tibana / MNRJ/2239 (MNRJ).

74
Oxysarcodexia floricola Lopes, 1975
(Appendix 17-B)
Type locality. Costa Rica, Guanacaste, 24 km NW Canas. Lopes, 1975e: 487.
Depository of type material. Unknown [original description only mentioned material
examined belonging to Dr. E. R. Heithaus].
Diagnosis. Male. Postocular plate with golden microtomentum. Ocellar setae not well-
developed. Thorax and abdomen with pale golden microtomentum. Dorsocentral post-sutural
setae with 2 well differentiated and 2 anterior setae smaller. Apical scutellar seta absent. Legs
blackish. T3 with 1 lateral marginal seta. Cerci, in lateral view, sinuous with normal (i.e. same
size as median area) apexes of oblique upwards edges. Setae ventrally on the cerci (lateral
view) present on apical ⅓ portion. Apical shape of the cerci (last ⅓ portion of the cercus; in
posterior view) with the same size as the middle part. Conformation of the cerci in posterior
view is parallel. Presence of a remarkable constriction at middle portion of the cerci, in
posterior view. In lateral view, distiphallus with smooth margins ventroapically; distiphallic
apical shape conic and straight distiphallic dorsal shape. Pregonite with expanded base
narrowing smoothly until the apex. Postgonite with expanded base and sudden narrowing at
apex. Vesica symmetric; terminal lobes well-developed, with filamentous at most tapering to
apex shape, sclerotized texture and presence of spines only along ventral edge; and presence of
an angular median projection of the main vesica branch.
Remarks. Specimen examined presents a damaged abdomen, lacking T4 (partially),
T5 and ST5 (both completely). Terminalia is on a microtube with glycerin. In original
description is mentioned yellow color for T5; T4 without median marginal seta presence and
there is no remarks for ST5 (Lopes 1975e). See “remarks” section on O. cyaniforceps for
further discussion.
Distribution. NEOTROPICAL. Brazil (Santa Catarina), Costa Rica (Guanacaste).
Biology. Unknown. Information available is only about the collection, in flowers
(Lopes 1975e).
Material examined. ♂: Nova Teutonia 27°11‟S, 52°23‟W Brazil, 300–500m 15-III
1968 Fritz Plaumann / Oxysarc. floricola ♂ Lopes Det. H. S. Lopes (MNRJ).
Oxysarcodexia fluminensis Lopes, 1946

75
(Appendix 17-C)
Type locality. Brazil, Rio de Janeiro, Guanabara, Grajaú. Lopes, 1946b: 104.
Depository of type material. MNRJ.
Diagnosis. Male. Postocular plate with golden microtomentum. Ocellar setae well-
developed. Thorax as well as abdomen with silvery microtomentum. Dorsocentral post-sutural
setae with 2 well differentiated and 1–3 anterior setae smaller. Apical scutellar seta present.
Legs blackish. T4 with 1 median marginal and 1 lateral marginal seta. T5 with golden
microtomentum in part. ST5 with parallel cleft edges and with setosity. Cerci, in lateral view,
straight with expanded apexes of oblique upwards edges. Setae ventrally on the cerci (lateral
view) present in apical ⅓ portion. Apical shape of the cerci (last ⅓ portion of the cercus; in
posterior view) smaller than the middle part. Conformation of the cerci in posterior view is
divergent. In lateral view, distiphallus with ventroapical concavity with serrated margins
ventroapically; presence of a lateroapical furrow. Distiphallic apical shape rounded and
sinuous distiphallic dorsal shape. Pregonite with expanded base narrowing smoothly until the
apex, which is darker than the base. Postgonite with expanded base and sudden narrowing at
apex. Vesica with symmetrical branches; terminal lobes well-developed, filamentous at most
tapering to apex shape, sclerotized texture and with presence of lateral lobes (i.e. division of
the vesica coming from or close to the basal branch of the vesica, placed laterally to phallic
tube); presence of spines only along the edges; and presence of a rounded median projection
of the main vesica branch.
Remarks. Distiphallus with a noteworthy concavity of serrated edges ventroapically.
Female was classified by Tibana & Mello (1985), according to the shape of the T6+7, as
syntergite partially divided into two plates.
Distribution. NEOTROPICAL. Brazil (Distrito Federal, Mato Grosso, Mato Grosso
do Sul, Minas Gerais, Pernambuco, Rio de Janeiro, São Paulo), Colombia (Antioquia).
Biology. Species already reared under laboratory conditions (Lopes 1973b), using dog
and primate feces, mouse carcass, crab (D‟Almeida 1989) and fish (D‟Almeida 1986) as
rearing substrates. Adults have been collected associated to pig carcass (Barros et al. 2008;
Cruz 2008; Barbosa et al. 2009) and cadavers (Oliveira-Costa et al. 2001). D‟Almeida (1984)
collected this fly using fish, bovine liver and human feces (decreasing scale of abundance) as
baits and pointed out a high synanthropic index for it. Baits as human feces, pig carcass, cow

76
liver and lung, chicken viscera, fish, crab, squid, rotten banana plus brown sugar and rotten
Syagrus comosa (Mart.) Mart. (Arecaceae), a species of a palmtree (at coastal area) has been
used for collect this species (Lopes 1973b; D‟Almeida & Lima 1994; Pamplona et al. 2000;
Oliveira et al. 2002; Leandro & D‟Almeida 2005; Vasconcelos & Araújo 2012; Ramírez-Mora
et al. 2012). Adults were already collected actively or using baited or W.O.T. traps at a forest
environment, an urban area and at Rio de Janeiro Zoological Garden (Pamplona et al. 2000;
Oliveira et al. 2002; Vasconcelos & Araújo 2012).
Material examined. ♂: Oxysarcodexia fluminensis sp 7 / COLOMBIA: Antioquia,
Copacabana, Ankon, 06°22‟1”N 75°29‟22,3”W 1.417m 4.viii.2010 Tr VSR 09:35 M. A.
Ramírez, I. Cadavid, C. Rave, Leg M08CP1 sp 7 (CE-TdeA).
Oxysarcodexia fraterna (Lopes, 1946)
Oxysarcodexia titubata ssp. fraterna Lopes, 1946
(Appendix 18-A)
Type locality. Mexico, Morelos, Cuernavaca. Lopes, 1946c: 143.
Depository of type material. MNRJ.
Diagnosis. Male. Postocular plate with golden microtomentum. Ocellar setae small,
but well-differentiated. Thorax grayish. Dorsocentral post-sutural setae with 3 well-
differentiated setae. Apical scutellar seta is present. Legs blackish. Abdomen grayish with
golden microtomentum more intense on T5. T4 with 1 pair of median marginal setae. ST5
reddish with a deep cleft. Cerci strongly bent backwards. Distiphallus with a large ventral lobe
and numerous pre-apical spines. This is a synopsis of the original description given by Lopes
(1946c).
Remarks. Cerci, in posterior view, convergent and with enlarged apexes, ventral
spines more developed and apex structural conformation of distiphallus are characteristics
used for differentiation of the very close related species O. peruviana and O. vittata (Lopes
1946c). See also “remarks” section on Oxysarcodexia n. sp. 4 for further discussion.
Distribution. NEARTIC. Mexico (Morelos).
Biology. Unknown. Information available is only about the type of collection, using
traps for collecting fruit flies (Lopes 1946c).

77
Material examined. ♂ terminalia mounted on a slide: O. titubata fraterna Lop №
8.101 cx: 55 Det. H. S. Lopes (MNRJ).
Oxysarcodexia fringidea (Curran & Walley, 1934)
Sarcophaga fringidea Curran & Walley, 1934
Oxysarcodexia lopesi Dodge, 1966
(Appendix 18-B)
Type locality. Guyana, Georgetown. Curran & Walley, 1934: 488.
Depository of type material. AMNH.
Diagnosis. Male. Postocular plate with pale golden microtomentum. Ocellar setae not
well-developed. Thorax with pale golden microtomentum more evident at humeral portion.
Dorsocentral post-sutural setae with 2 well differentiated and 1–3 anterior setae smaller.
Apical scutellar seta absent. Legs blackish. Abdomen with pale golden microtomentum. T3
with 3 lateral marginal setae. T4 with 1 median marginal and 3 lateral marginal setae. T5 with
golden microtomentum along the entire extension. ST5 with parallel cleft edges and with setae
on the apex of the arms. Cerci, in lateral view, straight with expanded apexes of oblique
upwards edges. Setae ventrally on the cerci (lateral view) present in apical ⅓ portion. Apical
shape of the cerci (last ⅓ portion of the cercus; in posterior view) with same size as the middle
part. Conformation of the cerci in posterior view is parallel. In lateral view, distiphallus with
ventroapical concavity with smooth margins ventroapically; distiphallic apical shape conic and
straight distiphallic dorsal shape. Pregonite with expanded base narrowing smoothly until the
apex, which is darker than the base, as well as the postgonite, except for the color which is the
same along the entire extension. Vesica with asymmetrical branches; terminal lobes well-
developed, with rounded shape and partially membranous texture; presence of spines only
along the edges; and presence of an angular median projection of the main vesica branch.
Remarks. Lopes (1938) redescribed this species and pointed out the occurrence of
intraspecific variation on the size of the vesica (reduced sometimes), although the asymmetry
is always observed. See “remarks” section on O. aurata for further discussion. Tibana &
Mello (1985) classified O. fringidea female, according to the shape of the T6+7, as syntergite
membranous.

78
Distribution. NEOTROPICAL. Bolivia (Cochabamba), Brazil (Amapá, Amazonas,
Bahia, Colombia, Espírito Santo, Maranhão, Mato Grosso, Minas Gerais, Pará, Pernambuco,
Rio de Janeiro), Colombia, Guyana (Georgetown, Kartabo), Peru (Camchamayo, Tingo
Maria), Venezuela (Caracas, Aragua).
Biology. Human feces are indicated as a rearing substrate under natural conditions. At
laboratory, O. fringidea was reared on agar plus powder milk, developing from 1st instar to
adults in 14–17 days (Lopes 1973b). Human feces, cow liver and lung, fish and squid baits
have already been used for collect (Curran & Walley 1934; Couri et al. 2000; Sousa et al.
2011). Methods of collection reported are actively, with nets, or using baited or Shannon traps
(Couri et al. 2000; Pamplona et al. 2000).
Material examined. ♂: COLOMBIA: Amazonas, PNN Amacayacu Camino a San
Martin, 3°41‟N 70°15‟W, 1-10.iii.2004, sweepnet, T. Pape & D. Arias. Id#4325 / NRM-DIPT
0014659 (NRM).
Oxysarcodexia galeata (Aldrich, 1916)
Sarcophaga galeata Aldrich, 1916
(Appendix 18-C)
Type locality. USA, Indiana, Lafayette. Aldrich, 1916: 280.
Depository of type material. USNM.
Diagnosis. Male. Postocular plate with silveryish microtomentum. Ocellar setae well-
developed. Thorax blackish with pale golden microtomentum more evident at humeral
portion. Dorsocentral post-sutural setae with 2 well differentiated and 1–3 anterior setae
smaller. Apical scutellar seta absent. Legs brownish. Abdomen blackish with some shades of
pale golden microtomentum. T3 with 1 lateral marginal seta. T4 with no median marginal and
1 lateral marginal seta. T5 with golden microtomentum along the entire extension, although
more intense at posterodorsal edge. ST5 with V-shaped cleft edges and with setosity and setae
on the apex of the arm. Cerci, in lateral view, sinuous with pointed apexes of oblique upwards
edges. Setae ventrally on the cerci (lateral view) present in apical ⅓ portion. Apical shape of
the cerci (last ⅓ portion of the cercus; in posterior view) with the same size as the middle part.
Conformation of the cerci in posterior view is parallel. Presence of a remarkable constriction
at middle portion of the cerci, in posterior view. In lateral view, distiphallus with smooth

79
margins ventroapically; distiphallic apical shape conic and straight distiphallic dorsal shape.
Presence of a large dorsoapical swelling on the distiphallus in lateral view. Lateroapical
expansions on distiphallus are present, in dorsal view. Pregonite with expanded base
narrowing smoothly until the apex, which is darker than the base. Postgonite with expanded
base and sudden narrowing at apex. Vesica with symmetrical branches; terminal lobes
reduced, with filamentous at most tapering to apex shape and sclerotized texture; presence of
spines along the edges; and presence of a rounded median projection of the main vesica
branch.
Remarks. See “remarks” section on O. aurata. Larvae of 1st, 2
nd and 3
rd instars were
described by Knipling (1936).
Distribution. NEARTIC. USA (Arkansas, Florida, Georgia, Illinois, Indiana, Iowa,
Kansas, New York, Tenessee, Texas).
Biology. Oxysarcodexia galeata has been collected in high lands (Dodge & Seago
1954) and in a wooded area, associated to a dog carcass (Reed 1958). Immatures normally
feed on excrement, however larvae obtained from one female were reared on decaying
hamburger meat and completed the development from larva to adult in 14 days (Knipling
1936).
Material examined. ♂: ALABAMA: Madson Co. 10km NE Maysville, Sharp Core,
Sneed Spg. Bottomland, 240m, 6 oct 1992, Acciavatti / NRM-DIPT 0014309 (NRM).
Oxysarcodexia grandis Lopes, 1946
(Appendix 19-A)
Type locality. Brazil, Rio de Janeiro, Angra dos Reis. Lopes, 1946b: 82.
Depository of type material. MNRJ.
Diagnosis. Male. Postocular plate with golden microtomentum. Ocellar setae well-
developed. Thorax with intense golden microtomentum, as well as the abdomen. Dorsocentral
post-sutural setae with 3 well differentiated and 1 smaller seta among these can be present.
Apical scutellar seta present. Legs blackish. T4 with 1 lateral marginal seta. T5 with golden
microtomentum along the entire extension. ST5 with parallel cleft edges and with setosity.
Cerci, in lateral view, straight with pointed apexes of oblique upwards edges. Setae ventrally
on the cerci (lateral view) present in apical ⅓ portion. Apical shape of the cerci (last ⅓ portion

80
of the cercus; in posterior view) with the same size as the middle part. Conformation of the
cerci in posterior view is parallel. In lateral view, distiphallus with ventroapical concavity with
smooth margins ventroapically; presence of a lateroapical furrow; distiphallic apical shape
rounded and sinuous distiphallic dorsal shape. Pregonite with expanded base and sudden
narrowing at apex with same color along the entire extension. Postgonite with expanded base
narrowing smoothly until the apex. Vesica with symmetrical branches; presence of lateral
lobes (i.e. division of the vesica coming from or close to the basal branch of the vesica, placed
laterally to phallic tube); terminal lobes well-developed, with filamentous at most tapering to
apex shape and sclerotized texture; presence of spines only on ventral surface; and presence of
a rounded median projection of the main vesica branch.
Remarks. Lopes (1946b) reported that dorsocentral post-sutural setae can, even for
species of a same colony, present variable number: 3 or 4 setae being the first 3 equidistant
and a smaller seta can be present among them. See also “remarks” section on O. augusta for
further discussion. Female was classified by Tibana & Mello (1985), according to the shape of
the T6+7, as syntergite undivided.
Distribution. NEOTROPICAL. Brazil (Paraná, Rio de Janeiro, São Paulo), Colombia
(Antioquia), Ecuador, Peru (Arequipa, San Miguel).
Biology. This species has already been reared under laboratory conditions (Lopes
1973b), although further information of the development was not specified. Attraction by
human feces, chicken viscera and fish head is documented (Lopes 1973b; Ramírez-Mora et al.
2012). In a study of synanthropy of Sarcophagidae from Colombia carried out by Yepes-
Gaurisas et al. (2013), it is reported the attraction of O. grandis mainly by chicken viscera,
collecting specimens both in rural and in forest areas, although this fly has been considered
infrequent.
Material examined. ♂♂: O. grandis SP 3; Oxysarcodexia, Det. Alejandro Ramírez M
2010 / COLOMBIA: Antioquia, Medellín, Cola del Zorro, 06°22‟19,7”N 75°32‟43,9”W
1.943m 10.iii.2010 Tr VSR 10:05 Col. M. A. Ramírez / T. de A. 1132 (CE-TdeA) //
COLOMBIA: Antioquia, Medellín, Cola del Zorro, 06°22‟19,7”N 75°32‟43,9”W 1.943m
10.iii.2010 Tr VSR 10:05 Col. M. A. Ramírez / T. de A. 1132 / O. grandis (CE-TdeA).
Oxysarcodexia inflata Lopes, 1975

81
(Appendix 19-B)
Type locality. Brazil, Maranhão, Rosário, Igarapé, Paraqueú. Lopes 1975c: 470.
Depository of type material. MNRJ.
Diagnosis. Male. Postocular plate with silvery microtomentum. Ocellar setae not well-
developed. Thorax and abdomen with pale golden microtomentum, slightly more intense on
the abdomen. Dorsocentral post-sutural setae with 2 well differentiated and 2 anterior setae
smaller. Apical scutellar seta present. Legs brownish. T3 with 1 lateral marginal seta. T4 with
1 median marginal and 2 lateral marginal setae. T5 with golden microtomentum along the
entire extension. ST5 with parallel cleft edges and with setosity and setae on the apex of the
arms. Cerci, in lateral view, sinuous with expanded apexes of oblique upwards edges. Setae
ventrally on the cerci (lateral view) present in full extension. Apical shape of the cerci (last ⅓
portion of the cercus; in posterior view) smaller than the middle part. Conformation of the
cerci in posterior view is parallel. In lateral view, distiphallus with serrated margins
ventroapically and ventroapical projections; distiphallic apical shape rounded and straight
distiphallic dorsal shape. In dorsal view, presence of lateroapical expansions on distiphallus.
Pregonite with expanded base and sudden narrowing at apex, which is darker than the base.
Postgonite with expanded base narrowing smoothly until the apex and same color along the
entire extension. Vesica with symmetrical branches and presence of lateral lobes (i.e. division
of the vesica coming from or close to the basal branch of the vesica, placed laterally to phallic
tube); terminal lobes well-developed, with filamentous at most tapering to apex shape and
sclerotized texture; the median area of terminal lobes more expanded than basal and apical
areas; presence of spines only on ventral surface and presence of a rounded median projection
of the main vesica branch.
Remarks. See “remarks” section on O. amorosa. Female was described by Lopes
(1975c) as presenting ocellar setae well-developed, absence of apical scutellar setae and, as
seen in O. amorosa female, T7 weakly sclerotized on the center and with black setosity.
Distribution. NEOTROPICAL. Brazil (Maranhão, Pará, Pernambuco).
Biology. Unknown.
Material examined. ♂: Igarapé PARAQUEÚ Rosário, MARANHÃO BRASIL /
20/22-XI-70 / H. Berla / Paratype / Oxysarcodexia inflata n.sp. ♂ Det. H. S. Lopes (MNRJ).

82
Oxysarcodexia injuncta (Walker, 1857)
Sarcophaga injuncta Walker, 1857
Oxysarcodexia plaumanni Lopes, 1946
(Appendix 19-C)
Type locality. Brazil, São Paulo, Eugênio Lefevre. Lopes, 1946b: 102.
Depository of type material. BMNH.
Diagnosis. Male. Postocular plate with golden microtomentum. Ocellar setae not well-
developed. Thorax with intense golden microtomentum. Dorsocentral post-sutural setae with 2
well differentiated and 1–3 anterior setae smaller. Apical scutellar seta present. Legs blackish.
Abdomen with silvery microtomentum. T3 with 2 lateral marginal setae. T4 with 1 median
marginal and 2 lateral marginal setae. T5 without golden microtomentum, only silvery
microtomentum is seen. ST5 with parallel cleft edges and with setae scattered on arm‟s
surface. Cerci, in lateral view, straight with expanded apexes of oblique upwards edges. Setae
ventrally on the cerci (lateral view) present in full extension. Apical shape of the cerci (last ⅓
portion of the cercus; in posterior view) smaller than the middle part. Conformation of the
cerci in posterior view is divergent. In lateral view, distiphallus with ventroapical concavity
with serrated margins ventroapically; presence of a lateroapical furrow; distiphallic apical
shape rounded and straight distiphallic dorsal shape. Lateroapical expansions, in dorsal view,
present on the distiphallus. Pregonite with expanded base and sudden narrowing at apex and
same color along the entire extension. Postgonite with expanded base narrowing smoothly
until the apex. Vesica with symmetrical branches; presence of lateral lobes (i.e. division of the
vesica coming from or close to the basal branch of the vesica, placed laterally to phallic tube);
terminal lobes well-developed, with filamentous at most tapering to apex shape and
sclerotized texture; presence of spines on both ventral and dorsal surfaces; and presence of a
rounded median projection of the main vesica branch.
Remarks. Oxysarcodexia injuncta is close related to O. paulistanensis, differing by the
golden microtomentum in the thorax, the straight dorsal shape of the phallus, the ventroapical
distiphallus shape, the vesica constitution and the apical cerci shape (Lopes 1946b). Female
was described by Lopes (1975b) and was classified by Tibana & Mello (1985), according to
the shape of the T6+7, as syntergite undivided. See also “remarks” section on O. bikini for
further discussion.

83
Distribution. NEOTROPICAL. Brazil (Mato Grosso, Mato Grosso do Sul, Minas
Gerais, Paraná, Rio de Janeiro, Rio Grande do Sul, Santa Catarina, São Paulo).
Biology. This fly has already been reared under laboratory conditions, although no
further information about its development was found on the literature, except the
attractiveness by human feces (Lopes 1973b). Occurrence of this species is basically
associated to highlands, as pointed out by Lopes (1973b) and seen in our collection, once O.
injuncta was only collected in high hills areas (Serra do Japi, in São Paulo state, and Serra do
Lopo, in Minas Gerais state, both in Brazil).
Material examined. ♂♂: Rio Japura / Amazon Roman / april / Oxysarcodexia pr.
plaumanni, Lopes, Det. H. S. Lopes / NRM-DIPT 0014313 (NRM) // BRAZIL: São Paulo /
Jundiaí, Serra do Japi, / 06.I.2012 / M. D. Grella (L2B-DBA) // BRAZIL: Minas Gerais, /
Extrema, Serra do Lopo, / Pedra das Flores, / 27.II.2012 / A. G. Savino, M. P. Nassu, M. D.
Grella (L2B-DBA).
Oxysarcodexia insolita Lopes, 1946
(Appendix 20-A)
Type locality. Guuyana, Esequibo River, Moraballi Creek. Lopes, 1946b: 89.
Depository of type material. BMNH.
Diagnosis. Male. Postocular plate with pale golden microtomentum. Ocellar setae not
well-developed. Thorax and abdomen with golden microtomentum more evident laterally.
Dorsocentral post-sutural setae with 2 well differentiated and 1–3 anterior setae smaller.
Apical scutellar seta present. Legs brownish. T3 with 2 lateral marginal setae. T4 with 1
median marginal and 2 lateral marginal setae. T5 with golden microtomentum in part. ST5
with parallel cleft edges and with setosity and setae scattered on arm‟s surface. Cerci, in lateral
view, sinuous with expanded apexes of straight edges. Setae ventrally on the cerci (lateral
view) present in apical ⅓ portion. Apical shape of the cerci (last ⅓ portion of the cercus; in
posterior view) bigger than the middle part. Conformation of the cerci in posterior view is
parallel. Presence of a remarkable constriction at middle portion of the cerci, in posterior view.
In lateral view, distiphallus with serrated margins ventroapically; distiphallic apical shape
rounded and straight distiphallic dorsal shape. Pregonite with expanded base narrowing
smoothly until the apex, which is darker than the base, as well as the postgonite, except for the

84
color which is the same along the entire extension. Vesica with symmetrical branches;
terminal lobes well-developed, with filamentous at most tapering to apex shape and
sclerotized texture; basal area of terminal lobes more expanded than the apical area; presence
of spines only on ventral surface; and presence of a rounded median projection of the main
vesica branch.
Remarks. Cerci, surstylus and phallus enable distinguish O. insolita from
Oxysarcodexia major Lopes, 1946, considered a close species. On distiphallus, ventroapically,
there is a serrated edge on O. insolita and a small group of spines on O. major, besides the
vesica, which is not long as seen in O. major, and presents long spines and a long and curved
basal area, absent on O. major whose spines are shorter (Lopes 1946b). Oxysarcodexia
petropolitana Lopes, 1975 is considered similar to O. insolita and O. major due to the vesica
shape, differing mostly by distiphallus apex and cerci shape (Lopes 1975c). Female was
classified by Tibana & Mello (1985), according to the shape of the T6+7, as syntergite
undivided.
Distribution. NEOTROPICAL. Brazil (Pará), Guyana (Moraballi Creek), Mexico
(Chiapas, Veracruz), Trinidad and Tobago (Trinidad).
Biology. Under laboratory conditions, O. insolita was reared on agar plus powder milk,
developing from first instar to adults in 14–17 days. Under natural conditions, it has been
reared on human feces (Lopes 1973b).
Material examined. ♂: ECUADOR: Napo Povince; Yasuní National Park; Yasuní
Research Station; 76°36‟W 00°38‟S; 3–20 XI 1998: T. Pape & B. Viklund / NRM-DIPT
0014317 (NRM).
Oxysarcodexia intona (Curran & Walley, 1934)
Sarcophaga intona Curran & Walley, 1934
Sarcophaga intonsa: Lopes (1969), incorrect subsequent spelling of intona Curran &
Walley, 1934
(Appendix 20-B)
Type locality. Guyana, Kartabo. Curran & Walley, 1934: 489.
Depository of type material. AMNH.

85
Diagnosis. Male. Postocular plate with golden microtomentum. Ocellar setae not well-
developed. Thorax and abdomen with silvery microtomentum. Dorsocentral post-sutural setae
with 2 well differentiated and 1–3 anterior setae smaller. Apical scutellar seta absent. Legs
brownish. T4 with no median marginal and 3 lateral marginal setae. T5 with golden
microtomentum, although not seen along the entire extension. ST5 with parallel cleft edges
and with setae on the apex of the arms. Cerci, in lateral view, sinuous with expanded apexes of
concave edges. Setae ventrally on the cerci (lateral view) present in apical ⅓ portion. Apical
shape of the cerci (last ⅓ portion of the cercus; in posterior view) with same size as the middle
part. Conformation of the cerci in posterior view is divergent. In lateral view, distiphallus with
smooth margins ventroapically; presence of ventroapical projections and lateral lobes;
distiphallic apical shape rounded and sinuous distiphallic dorsal shape. A large dorsoapical
swelling in the distiphallus (lateral view) is present. Pregonite with expanded base and sudden
narrowing at apex, which is darker than the base, as well as the postgonite, except for the color
which is the same along the entire extension. Vesica with symmetrical branches; terminal
lobes reduced, with filamentous at most tapering to apex shape and partially membranous
texture; presence of spines on both ventral and dorsal surfaces; and presence of an angular
median projection of the main vesica branch.
Remarks. See “remarks” section on O. aurata. Female was classified by Tibana &
Mello (1985), according to the shape of the T6+7, as syntergite membranous.
Distribution. NEOTROPICAL. Brazil (Amazonas, Amapá, Ceará, Espírito Santo,
Maranhão, Minas Gerais, Pará, Pernambuco, Rio de Janeiro), Guyana.
Biology. This fly has been collected associated to pig and rat carcasses, in Brazilian
Cerrado (“savanna-like”) vegetation, forest environments and an urban area (Cruz 2008;
Barbosa et al. 2009; Vasconcelos & Araújo 2012). Baits as feces, banana plus brown sugar,
rotting liver, cow liver and lung, fish and squid has been used with traps (W.O.T. or “can”
traps) (Lopes 1975a; Pamplona et al. 2000; Oliveira et al. 2002).
Material examined. ♂♂: RIO DE JANEIRO, BRASIL / R. Tibana / NRM-DIPT
0014318 (NRM) // Guarapari, Esp. Santo, Brasil / H. S. Lopes, 9.I.75 / Oxysarcodexia intona
♂ (C. et w.), det. H. S. Lopes (ZMUC).
Oxysarcodexia jamesi Dodge, 1968

86
(Appendix 20-C)
Type locality. Panama, Canal Zone, Barro Colorado Island. Dodge, 1968: 433.
Depository of type material. KU.
Diagnosis. Male. Postocular plate with golden microtomentum. Ocellar setae well-
developed. Thorax and abdomen with golden microtomentum. Dorsocentral post-sutural setae
with 3 well differentiated setae and 1 anterior seta smaller. Apical scutellar seta present. Legs
brownish. T3 with 2 lateral marginal setae. T4 with 2 median marginal and 3 lateral marginal
setae. ST5 with parallel cleft edges and setosity. Cerci, in lateral view, straight with expanded
apexes of oblique upwards edges. Setae ventrally on the cerci (lateral view) present in full
extension. Apical shape of the cerci (last ⅓ portion of the cercus; in posterior view) smaller
than the middle part. Conformation of the cerci in posterior view is parallel. In lateral view,
distiphallus with membranous ventroapical concavity of smooth margins; distiphallic apical
shape conic and sinuous dorsal shape. Presence of a notable dilatation on the distiphallus
lateroapically. Pregonite with base and apex with same size and same color along the entire
extension. Postgonite with expanded base and sudden narrowing at apex and same color along
the entire extension. Vesica symmetric; terminal lobes reduced, with filamentous at most
tapering to apex shape, partially membranous texture and with micro-spines only on ventral
surface; presence of a rounded median projection of the main vesica branch.
Remarks. Cerci and somehow the distiphallus apical concavity are similar to those
present in Oxysarcodexia peculiaris Lopes, 1975, although vesica is very unlike. See also
“remarks” section on O. augusta for further discussion.
Distribution. NEOTROPICAL. Panama (Barro Colorado Island), Mexico.
Biology. Unknown. This fly specimen was collected from over a swarm raid of Eciton
burchelli (Westwood, 1842), an ant species (Dodge 1968).
Material examined. ♂: Santa Rosa National Park, Guanacaste Prov. COSTA RICA
Nov. 1998 300m D. H. Jansen & W. Hallwachs / Oxysarcodexia jamesi Dodge Det. H. S.
Lopes (MNRJ).
Oxysarcodexia major Lopes, 1946
(Appendix 21-A)
Type locality. Brazil, Rio de Janeiro, Guanabará, Grajaú. Lopes, 1946b: 88.

87
Depository of type material. MNRJ.
Diagnosis. Male. Postocular plate with golden microtomentum. Ocellar setae not well-
developed. Thorax and abdomen with intense golden microtomentum more evident laterally.
Dorsocentral post-sutural setae with 2 well differentiated and 1–3 anterior setae smaller.
Apical scutellar seta present. Legs blackish. T3 with 2 lateral marginal setae. T4 with 1
median marginal and 2 lateral marginal setae. T5 with golden microtomentum in part. ST5
with parallel cleft edges and with setosity and setae scattered on arm‟s surface. Cerci, in lateral
view, sinuous with normal apexes (i.e. same size as median area) of oblique upwards edges.
Setae ventrally on the cerci (lateral view) absent only in the middle portion. Apical shape of
the cerci (last ⅓ portion of the cercus; in posterior view) with the same size as the middle part.
Conformation of the cerci in posterior view is parallel. Presence of a remarkable constriction
at middle portion of the cerci, in posterior view. In lateral view, distiphallus with serrated
margins ventroapically; distiphallic apical shape rounded and straight distiphallic dorsal shape.
Pregonite with expanded base and sudden narrowing at apex, which is darker than the base, as
well as the postgonite, except for the color which is the same along the entire extension.
Vesica with symmetrical branches; terminal lobes well-developed, elongated, with
filamentous at most tapering to apex shape and sclerotized texture; presence of spines only on
ventral surface; and presence of an angular median projection of the main vesica branch.
Remarks. See “remarks” section on O. insolita. Female was classified by Tibana &
Mello (1985), according to the shape of the T6+7, as syntergite undivided.
Distribution. NEOTROPICAL. Brazil (Amazonas, Amapá, Mato Grosso, Rio de
Janeiro, Roraima), Colombia (Antioquia), Ecuador, El Salvador, Mexico (Veracruz), Peru,
Trinidad and Tobago (Trinidad).
Biology. Collections of this species have been done with human feces, rotten Syagrus
comosa (Mart.) Mart. (Arecaceae), a species of a palmtree (at coastal area), rotten banana and
brown sugar, rotting liver, rotting beef lung, chicken viscera and fish head as baits (Lopes
1973b; Oliveira et al. 2002; Sousa et al. 2011; Ramírez-Mora et al. 2012). The methods of this
collections included flytraps, W.O.T., malaise and Shannon traps (Lopes & Tibana 1991;
Oliveira et al. 2002; Sousa et al. 2011). Oxysarcodexia major has already been reared under
laboratory conditions (Lopes 1973b), even though no detailed information about its
development had been reported.

88
Material examined. ♂: Oxysarcodexia bakeri? SP 8 / TdeA 1311 [from Colombia]
(CE-TdeA).
Oxysarcodexia marina (Hall, 1938)
Apelophyla marina Hall, 1938
(Appendix 21-B)
Type locality. Argentina, Mendoza. Hall, 1938: 257.
Depository of type material. DEI.
Diagnosis. Male. Postocular plate with golden microtomentum. Ocellar setae well-
developed. Thorax and abdomen with silvery microtomentum. Three well differentiated
dorsocentral post-sutural setae. Apical scutellar seta absent. Legs blackish. T4 with presence
of small median marginal setae. ST5 with parallel cleft edges. Syntergosternite 7+8 black.
Cerci, in lateral view, bent backwards with expanded apexes of oblique upwards edges. Setae
ventrally on the cerci (lateral view) present in full extension. Apical shape of the cerci (last ⅓
portion of the cercus; posterior view) smaller than the middle part. Conformation of the cerci
in posterior view is divergent. Presence of a remarkable dilatation at middle portion of the
cerci, in posterior view. In lateral view, distiphallus with smooth margins ventroapically;
presence of lateral lobes and a large dorsoapical swelling; distiphallic apical shape
square/oblong and straight dorsal shape. Presence of lateroapical expansions on the
distiphallus, in dorsal view. Pregonite and postgonite with expanded base narrowing smoothly
until the apex and the same color along the entire extension. Vesica symmetric, with presence
of lateral lobes (i.e. division of the vesica coming from or close to the basal branch of the
vesica, placed laterally to phallic tube); terminal lobes well-developed, with square shape and
sclerotized texture; ventroapical area of terminal lobes notably elongated; presence of spines
along the edges (not continuously). This diagnosis is based on the examination of one
specimen (specified below) presenting some conservation issues (“oily” aspect compromising
original microtomentum and color, T5 partially broke and terminalia on glycerin lacking ST5)
and on diagnosis provided by Lopes (1975c).
Remarks. Male morphology and coloration of O. marina are considered similar to O.
paulistanensis, although O. marina presenting black color on syntergosternite 7+8 and
absence of apical scutellar setae (Mulieri et al. 2010) and we also pointed out the peculiarity

89
of O. marina terminalia seen on cerci shape and constitution of the vesica, especially the
ventroapical elongation. A good and description of female was given by Mulieri et al. (2010),
that also stress the sternites of female terminalia resemble in general shape and coloration
those of O. varia.
Distribution. NEOTROPICAL. Argentina (Buenos Aires, Mendoza), Brazil (Rio
Grande do Sul).
Biology. Attractiveness is reported by rotten bovine and cow liver and dog feces
(Mariluis et al. 2007; Mulieri et al. 2008; Mulieri et al. 2010; Mulieri et al. 2011). In Buenos
Aires, Argentina, O. marina has been collected in grassland and in rural areas (Mulieri et al.
2008; Mulieri et al. 2011). Adults of this fly are also considered flower visitors of Ludwigia
spp., a water primrose of Onagraceae family (Mulieri et al. 2010).
Material examined. ♂: Pelotas 7 Maio 1963 R. G. do Sul – Brasil C. M. Biezanko leg.
/ Apelophyla marina ♂ Hall Det. H. S. Lopes (MNRJ).
Oxysarcodexia meridionalis (Engel, 1931)
Sarcophaga (Dasyproctia) ventricosa var. meridionalis Engel, 1931
Sarcophaga illata Lopes, 1938
(Appendix 21-C)
Type locality. Argentina, Chaco, San José. Engel, 1931: 146.
Depository of lectotype species. SMN.
Diagnosis. Male. Postocular plate with golden microtomentum. Ocellar setae well-
developed. Thorax and abdomen with golden microtomentum, although a slightly silvery
microtomentum is seen ventrally on the abdomen. Dorsocentral post-sutural setae with 2 well
differentiated and 2 anterior setae smaller. Apical scutellar seta absent. Legs blackish. T3 with
2 lateral marginal setae. T4 with 1 median marginal and 3 lateral marginal setae. T5 with
golden microtomentum along the entire extension. ST5 with parallel cleft edges and with
setosity and setae on the apex of the arms. Cerci, in lateral view, straight with pointed apexes
of straight edges. Setae ventrally on the cerci (lateral view) present in apical ⅓ portion. Apical
shape of the cerci (last ⅓ portion of the cercus; in posterior view) smaller than the middle part.
Conformation of the cerci in posterior view is parallel. In lateral view, distiphallus with
smooth margins ventroapically; distiphallic apical shape rounded and sinuous distiphallic

90
dorsal shape. Pregonite and postgonite with expanded base narrowing smoothly until the apex
and same along the entire extension. Vesica with symmetrical branches; terminal lobes well-
developed, mediolateral expanded, with rounded shape, sclerotized texture and presence of
spines only on ventral surface.
Remarks. It is considered probably as a member of “ventricosa” group, although the
lack of female study forbids this statement (Lopes 1975c). Phallus of O. meridionalis is
similar to that of O. parva, although cerci present slender and more pointed shape and vesica
terminal lobes are bigger, completely sclerotized and adorned with spines.
Distribution. NEOTROPICAL. Argentina (Chaco, Delta Del Paraná, San José),
Bolivia, Brazil (Goiás, Mato Grosso, Mato Grosso do Sul, Minas Gerais, São Paulo).
Biology. Unknown. The only information of O. meridionalis is the occurrence in
Cerrado (“savanna-like”) environment (Lopes 1973b) and on an urban area of Campinas city,
São Paulo state, Brazil (data from our research group).
Material examined. ♂: BRAZIL: São Paulo, Campinas, UNICAMP, 16.VII.2012, D.
Brancoli, A. G. Savino / II – 16/7 ? O. meridionalis (L2B-DBA).
Oxysarcodexia mitifica Lopes, 1953
(Appendix 22-A)
Type locality. Venezuela, Caracas. Lopes, 1953: 50.
Depository of type material. AMNH.
Diagnosis. Male. Postocular plate with golden microtomentum. Ocellar setae well-
developed. Thorax and abdomen with golden microtomentum. Dorsocentral post-sutural setae
with 2 well differentiated and 1–3 anterior setae smaller. Apical scutellar seta absent. Legs
blackish. T3 with lateral marginal seta. T4 with 1 pair of median marginal and 2 lateral
marginal setae. T5 with golden microtomentum. ST5 with parallel cleft edges and with
setosity. Cerci, in lateral view, straight with pointed apexes of oblique upwards edges. Setae
ventrally on the cerci (lateral view) present in apical ⅓ portion. Apical shape of the cerci (last
⅓ portion of the cercus; in posterior view) with same size as the middle part. Conformation of
the cerci in posterior view is parallel. Presence of a remarkable constriction at middle portion
of the cerci, in posterior view. In lateral view, distiphallus with smooth margins
ventroapically; presence of a lateroapical furrow, distiphallic apical shape rounded and

91
sinuous distiphallic dorsal shape. Pregonite with expanded base narrowing smoothly until the
apex, which is darker than the base. Postgonite with expanded base and sudden narrowing at
apex. Vesica with symmetrical branches; terminal lobe well-developed, with filamentous at
most tapering to apex shape, flattened laterally and with partially membranous texture;
presence of spines on both dorsal and ventral surfaces; and presence of an angular median
projection of the main vesica branch.
Remarks. The main difference in comparison to other Oxysarcodexia species is the
vesica, which presents a great development of median lobe (ventroapically directed) while
lateral lobes (laterally directed) are small.
Distribution. NEOTROPICAL. Colombia (Antioquia), Costa Rica, Ecuador, El
Salvador, Panama (Barro Colorado Island), Peru, Venezuela (Caracas).
Biology. This fly specimen was collected from over a swarm raid of Eciton burchelli
(Westwood, 1842), an ant species (Dodge 1968). Chicken viscera and fish head were baits
used to attract and collect them in Antioquia, Colombia (Ramírez-Mora et al. 2012).
Material examined. ♂: COLOMBIA: SP21 4CP2 / O. ramosa? (CE-TdeA) [from
Antioquia].
Oxysarcodexia modesta Lopes, 1946
(Appendix 22-B)
Type locality. Brazil, Rio de Janeiro, Guanabara, Gávea. Lopes, 1946b: 129.
Depository of type material. MNRJ.
Diagnosis. Male. Postocular plate with pale golden microtomentum. Ocellar setae
well-developed. Thorax and abdomen with pale golden microtomentum. Dorsocentral post-
sutural setae with 2 well differentiated and 1–3 anterior setae smaller. Apical scutellar seta
absent. Legs brownish. T3 with 1 lateral marginal seta. T4 with 1 median marginal and 2
lateral marginal setae. T5 with golden microtomentum in part. ST5 with parallel cleft edges
and with setae on the apical half of the arms. Cerci, in lateral view, straight with pointed
apexes of oblique upwards edges. Setae ventrally on the cerci (lateral view) present in full
extension. Apical shape of the cerci (last ⅓ portion of the cercus; in posterior view) smaller
than the middle part. Conformation of the cerci in posterior view is divergent. In lateral view,
distiphallus with the presence of a ventroapical concavity with serrated margins

92
ventroapically; distiphallic apical shape rounded and sinuous distiphallic dorsal shape;
presence of ventroapical projections. Pregonite with expanded base and sudden narrowing at
apex and same along the entire extension. Postgonite with expanded base narrowing smoothly
until the apex. Vesica with symmetrical branches; terminal lobes well-developed, with
filamentous at most tapering to apex shape and partially membranous texture; presence of
spines on both dorsal and ventral surfaces; and presence of a rounded median projection of the
main vesica branch.
Remarks. See “remarks” section on O. bakeri. Female was classified by Tibana &
Mello (1985), according to the shape of the T6+7, as syntergite partially divided into two
plates.
Distribution. NEOTROPICAL. Brazil (Minas Gerais, Rio de Janeiro, Pará,
Pernambuco, São Paulo), Peru.
Biology. Under laboratory conditions, O. modesta was reared on agar plus powder
milk, developing from 1st instar to adults in 14–17 days (Lopes 1973b). Adults attractiveness
has been observed by human feces, pig and mouse carcasses, chicken viscera (including liver),
rotting cow liver, fish, banana plus brown sugar and rotten Syagrus comosa (Mart.) Mart.
(Arecaceae), a species of a palmtree (at coastal area) (Lopes 1973b; Linhares 1981; Dias et al.
1984c; Mendes & Linhares 1993; Oliveira et al. 2002; Cruz 2008; Barbosa et al. 2009;
Oliveira & Vasconcelos 2010; Rosa et al. 2011). Comparing the attractiveness of human feces,
chicken viscera and mouse carcass baits, Linhares (1981) observed no differences among
these three substrates for O. modesta. Moreover, a lower occurrence in urban zone in contrast
to rural and forest areas analyzed, pointing out a low correlation with the anthropobiocenosis.
Sunlight instead of shaded areas is also referred as a preference of this species (Linhares
1981). This species has been caught on the premises of an institute of legal medicine, attracted
by chicken liver and classified as a necrophagous/omnivore species (Oliveira & Vasconcelos
2010). Adults have also been collected associated to a zoological garden in Rio de Janeiro
state, Brazil (Oliveira et al. 2002) and in urban and forest environments of Brazilian
northeastern (Vasconcelos & Araújo 2012).
Material examined. ♂♂: Quinta da Bôa Vista, São Cristóvão, Rio, Brasil / R. Tibana
21. VI.73 / NRM-DIPT 0014319 (NRM) // Rio das Ostras, E. do Rio, Brasil / 15-I-96, E. S.

93
Conrado col. / Oxysarcodexia modesta Lopes Det. R. Tibana (MNRJ) // Brasil: Amapá,
Mazagão, v–vi 1993 J. M. D‟Almeida col (MNRJ).
Oxysarcodexia molitor (Curran & Walley, 1934)
Sarcophaga molitor Curran & Walley, 1934
(Appendix 22-C)
Type locality. Guyana, Kaieteur. Curran & Walley, 1934: 488.
Depository of type material. AMNH.
Diagnosis. Male. Ocellar setae not well-developed. Thorax and abdomen grayish with
golden microtomentum more intense laterally on thorax and on T4. Dorsocentral post-sutural
setae with 2 well differentiated and 2 anterior smaller setae. Apical scutellar setae present,
although very small. Legs blackish. T5 yellowish. ST5 small, with setosity, parallel cleft edges
and with small triangular projection on the posterior part of the arms. Cerci robust with
truncated apex. Anterior area of the apical distiphallic plate largely membranous, with a pair
of spinous internal plates. Vesica very sclerotized with an apical membranous lobe very
reduced. This is a synopsis of the original description given by Curran & Walley (1934) and of
the redescription given by Lopes (1975c).
Remarks. See “remarks” section on O. confusa. Female was described by Curran &
Walley (1934), presenting the first genital segment reddish with golden microtomentum,
sparse black setosity and an apical fringe of setae creased above.
Distribution. NEOTROPICAL. Guyana (Kartabo).
Biology. Unknown. The only information is about the substrate, human excrement, in
which the type species was collected on (Curran &Walley 1934).
Oxysarcodexia morretesi Tibana & Mello, 1983
(Appendix 23-A)
Type locality. Brazil, Paraná, São José dos Pinhais, Castelhanos. Tibana & Mello,
1983b: 279.
Depository of type material. MNRJ.
Diagnosis. Male. Postocular plate with golden microtomentum. Ocellar setae not well-
developed. Thorax and abdomen with grayish microtomentum. Dorsocentral post-sutural setae

94
with 2 well differentiated and 3 anterior setae smaller. Apical scutellar seta absent. Legs
brownish. T3 with 2 lateral marginal setae. T4 with 1 median marginal and 3 lateral marginal
setae. T5 with golden microtomentum along the entire extension. ST5 with parallel cleft edges
and with setosity along the edges of the arms. Cerci, in lateral view, straight with normal
apexes (same size as median area) of oblique upwards edges, due to a slight curvature and
with the mediolateral margin coming outwards. Setae ventrally on the cerci (lateral view)
present in apical half. Apical shape of the cerci (last ⅓ portion of the cercus; in posterior view)
with the same size as the middle part. Conformation of the cerci in posterior view is parallel.
Presence of a remarkable constriction at middle portion of the cerci, in posterior view. In
lateral view, distiphallus with presence of a ventromedial elongation; with smooth margins
ventroapically; distiphallic apical rounded shape and straight dorsal shape; presence of a small
“finger-like” swelling dorsoapically. Pregonite with expanded base narrowing smoothly until
the apex, which is darker than the base. Postgonite with expanded base and sudden narrowing
at apex. Vesica with symmetrical branches; terminal lobes well-developed, with oblong shape
and sclerotized texture; presence of spines only along the edges; and presence of an angular
median projection of the main vesica branch.
Remarks. Similar to O. thornax due to the presence of the “finger-like” dorsoapical
swelling and vesica constitution. Differences are observed on cerci (with mediolateral margin
coming outwards and slight curved apex), distiphallus structures (with membranous
expansions extended ventrally and enlarged median process with apical spines) and vesica
(without presence of two enlarged lateral plates with serrated edges) (Tibana & Mello 1983b).
See also “remarks” section on O. conclausa for further discussion.
Distribution. NEOTROPICAL. Brazil (Rio de Janeiro, Paraná, São Paulo).
Biology. Unknown.
Material examined. ♂: Brazil, Município de Bertioga, S.P., 27–30 Nov. 72, B. V.
Peterson / Paratype / Oxysarcodexia ♂ n.sp. near thornax Det. H. S. Lopes (MNRJ).
Oxysarcodexia nitida Soares & Mello-Patiu, 2010
(Appendix 23-B)
Type locality. Peru: Avispas, Madre de Dios. Soares & Mello-Patiu, 2010: 72.
Depository of type material. MNRJ.

95
Diagnosis. Male. Postocular plate with pale golden microtomentum. Ocellar setae
well-developed. Thorax and abdomen with golden microtomentum. Dorsocentral post-sutural
setae with 3 well differentiated setae, although a small seta among these 3 can be present.
Apical scutellar seta present. Legs blackish. T3 with 2 lateral marginal setae. T4 with 1
median marginal and 2 lateral marginal setae. T5 with golden microtomentum in part. ST5
with parallel cleft edges and with setosity and setae on the apex of the arm. Cerci, in lateral
view, straight with pointed apexes of oblique upwards edges. Setae ventrally on the cerci
(lateral view) present at basal half portion. Apical shape of the cerci (last ⅓ portion of the
cercus; in posterior view) smaller than the middle part. Conformation of the cerci in posterior
view is divergent. In lateral view, distiphallus with smooth margins ventroapically; distiphallic
apical shape conic; straight distiphallic dorsal shape; and presence of lateral lobes. Presence of
lateroapical expansions on distiphallus in dorsal view. Pregonite with base and apex with same
size and the apex is darker than the base. Postgonite with expanded base narrowing smoothly
until the apex. Vesica with symmetrical branches; terminal lobes well-developed, with square
shape and sclerotized texture; without presence of spines.
Remarks. This species is considered very close morphologically to O. vittata, with
differences evident on vesica shape and adornment, distiphallus apex and pregonite (Soares &
Mello-Patiu 2010). In original description the apical scutellar is absent, although it is present
in the material here examined. See “remarks” section on Oxysarcodexia n. sp. 4 and O.
augusta for further discussion.
Distribution. NEOTROPICAL. Ecuador (Napo), Peru (Avispas).
Biology. Unknown.
Material examined. ♂: ECUADOR: Napo Province, Yasuní Research Station;
76°36‟W 00°38‟S; 3–20.XI.1998; T. Pape & B. Viklund / NRM-DIPT 0014471 (NRM).
Oxysarcodexia notata Soares & Mello-Patiu, 2010
(Appendix 23-C)
Type locality. Peru: Avispas, Madre de Dios. Soares & Mello-Patiu, 2010: 74.
Depository of type material. MNRJ.
Diagnosis. Male. Postocular plate with golden microtomentum. Ocellar setae well-
developed. Thorax and abdomen with golden microtomentum. Dorsocentral post-sutural setae

96
with 3 well differentiated setae, although a small seta among these 3 can be present. Apical
scutellar seta present. Legs brownish. T3 with 1 lateral marginal seta. T4 with 1 median
marginal and 2 lateral marginal setae. T5 with golden microtomentum along the entire
extension. ST5 with parallel cleft edges and with setosity and setae scattered on arm‟s surface.
Cerci, in lateral view, straight with pointed apexes of oblique upwards edges. Setae ventrally
on the cerci (lateral view) absent only in the middle portion. Apical shape of the cerci (last ⅓
portion of the cercus; in posterior view) smaller than the middle part. Conformation of the
cerci in posterior view is divergent. In lateral view, distiphallus with serrated margins
ventroapically; distiphallic apical shape square, sinuous distiphallic dorsal shape; and presence
of ventroapical projections and lateral lobes. Presence of lateroapical expansions on
distiphallus in dorsal view. Pregonite and postgonite with expanded base narrowing smoothly
until the apex and same color along the entire extension. Vesica with symmetrical branches;
terminal lobes well-developed, with filamentous at most tapering to apex shape and
sclerotized texture; presence of micro-spines both on dorsal and ventral surfaces; and presence
of a rounded median projection of the main vesica branch.
Remarks. Male terminalia is considered similar to that presented by O. xon, with
differences observed on vesica and distiphallus morphologies (Soares & Mello-Patiu 2010).
See “remarks” section on Oxysarcodexia n. sp. 4 and O. augusta for further discussion.
Biology. Unknown.
Distribution. NEOTROPICAL. Ecuador (Napo), Peru (Avispas).
Material examined. ♂: ECUADOR: Napo Province, Yasuní National Park, Yasuní
Research Station; 76°36‟W 00°38‟S; 3–20.XI.1998; T. Pape & B. Viklund / NRM-DIPT
0014466 (NRM).
Oxysarcodexia occulta Lopes, 1946
(Appendix 24-A)
Type locality. Brazil, Rio de Janeiro, Guanabara, Corcovado. Lopes, 1946b: 112.
Depository of type material. MNRJ.
Diagnosis. Male. Postocular plate golden microtomentum. Ocellar setae not well-
developed. Thorax and abdomen with intense golden microtomentum. Dorsocentral post-
sutural setae with 2 well differentiated and 1–3 anterior setae smaller. Apical scutellar seta

97
absent. Legs blackish. T3 with 1 lateral marginal seta. T4 with 1 median marginal and 2 lateral
marginal setae. T5 with golden microtomentum in part. ST5 with parallel cleft edges and with
setosity. Cerci, in lateral view, bent backwards with normal apexes (same size as median area)
of oblique upwards edges. Setae ventrally on the cerci (lateral view) present in apical ⅓
portion. Apical shape of the cerci (last ⅓ portion of the cercus; in posterior view) with the
same size as the middle part. Conformation of the cerci in posterior view is divergent. In
lateral view, distiphallus with smooth margins ventroapically; distiphallic apical shape
rounded and sinuous distiphallic dorsal shape. Pregonite with expanded base and sudden
narrowing at apex, which is darker than the base, as well as the postgonite, except for the color
which is the same along the entire extension. Vesica with symmetrical branches; terminal
lobes well-developed, with filamentous at most tapering to apex shape, flattened
anteroposteriorly and partially membranous texture; without spines; and with the presence of
an angular median projection of the main vesica branch.
Remarks. See “remarks” section on O. comparilis. Female was classified by Tibana &
Mello (1985), according to the shape of the T6+7, as syntergite undivided.
Biology. In laboratory, O. occulta was reared on agar plus powder milk, developing
from first instar to adults in 14–17 days (Lopes 1973b). In natural environment, adults has
been caught with banana and brown sugar as bait and larvae, obtained from one female of the
collected specimens, were reared in human feces, taken 4 days to reach 3rd
larval instar plus 13
days to emerge adults (Lopes 1975a). This fly has also been collected from over a swarm raid
of Eciton burchelli (Westwood, 1842), an ant species (Dodge 1968). Attractiveness is reported
by human feces and rotten banana plus brown sugar (Lopes 1973b). Methods of collection
used for this species include malaise and Shannon traps (Lopes & Tibana 1991), traps for
collecting butterflies (Lopes 1975a) and actively, using baits to attract them and sweepnet for
catching (our collection).
Distribution. NEOTROPICAL. Brazil (Ceará, Mato Grosso, Mato Grosso do Sul,
Pará, Rio de Janeiro, Roraima, São Paulo), Colombia, Ecuador, Panama (Barro Colorado
Island).
Material examined. ♂♂: BRAZIL: São Paulo, Mogi Guaçu, Campininha, 18.IX.2011,
C. G. P. Lima, M. D. Grella, N. M. Jimenez / Oxysarcodexia sp III, Mogi Guaçu-SP,
18/11/2011, 16 (L2B-DBA); BRAZIL: São Paulo, Mogi Guaçu, Campininha, 18.IX.2011, C.

98
G. P. Lima, M. D. Grella, N. M. Jimenez / Oxysarcodexia sp III, Mogi Guaçu-SP, 18/11/2011,
16 (L2B-DBA).
Oxysarcodexia orbitalis Dodge, 1966
(Appendix 24-B)
Type locality. Martinique, Mont Pélé, 2 km N Morne Range. Dodge, 1966: 686.
Depository of type material. USNM.
Diagnosis. Male. Postocular plate with golden microtomentum. Ocellar seta not well-
developed (“hair-like”). Presence of 1 proclinate frontorbital seta. Thorax black with
yellowish-gray microtomentum on humeral region and laterally. Four dorsocentral post-sutural
setae. Apical scutellar seta is absent. Legs black. Abdomen black with grayish
microtomentum. T3 without median marginal row of seta. ST5 with arms crossed internally.
Cerci elongated, fused to apexes, which are shining black bosses and with sinuous shape
(lateral view). Pregonite strongly curved and postgonite straighter. Phallus with usual lobes
and distally strongly compressed. Vesica symmetrical, short, “ear-shaped”, with bulbous base
(in lateral view, a hole in the middle) and spinous arms. This is a synopsis of original
description given by Dodge (1966).
Remarks. Oxysarcodexia orbitalis is the unique species of the entire genus referred
presenting proclinate frontorbital seta (Dodge 1966). See “remarks” section on O. cyanea for
further discussion.
Distribution. NEOTROPICAL. Martinique.
Biology. Unknown. The only information is about the collection of the type species,
made on guava (Dodge 1966).
Oxysarcodexia pallisteri Dodge, 1966
(Appendix 24-C)
Type locality. Peru, Machu Picchu, Pueblo Cusa. Dodge, 1966: 686.
Depository of type material. AMNH.
Diagnosis. Male. Postocular plate with pale golden microtomentum. Ocellar setae
well-developed. Thorax with pale golden microtomentum. Dorsocentral post-sutural setae
with 2 well differentiated and 2 anterior setae smaller. Apical scutellar seta present, although

99
small. Legs brownish. Abdomen with silvery microtomentum. T3 with 1 lateral marginal seta.
T4 with 1 median marginal and 2 lateral marginal setae. T5 without golden microtomentum,
only silvery microtomentum is present. ST5 with parallel cleft edges and with setosity and
setae on the apex of the arms. Cerci, in lateral view, sinuous with pointed apexes of straight
edges. Setae ventrally on the cerci (lateral view) absent in the middle portion. Apical shape of
the cerci (last ⅓ portion of the cercus; in posterior view) smaller than the middle part.
Conformation of the cerci in posterior view is divergent. In lateral view, distiphallus with
ventroapical concavity of smooth margins and presence of serrated margins ventroapically;
presence of lateral lobes, ventroapical projections and medium dorsoapical swelling;
distiphallic apical shape square/oblong and sinuous distiphallic dorsal shape. Presence of
lateroapical expansions, in dorsal view. Pregonite and postgonite with expanded base
narrowing smoothly until the apex and same color along the entire extension. Vesica
symmetric; terminal lobes well-developed, with rounded shape, sclerotized texture and
presence of spines only on ventral surface.
Remarks. See “remarks” section on Oxysarcodexia n. sp. 4.
Distribution. NEOTROPICAL. Peru (Machu Pichu), Brazil (Paraná).
Biology. Unknown.
Material examined. ♂: IGUASSÚ Paraná XII - 941 Com. E. N. V. / Xarcophaga /
pallisteri (MNRJ).
Oxysarcodexia parva Lopes, 1946
(Appendix 25-A)
Type locality. BRASIL, RIO DE JANEIRO, Rio de Janeiro, Grajaú. Lopes, 1946b: 97.
Depository of type material. MNRJ.
Diagnosis. Male. Postocular plate with golden microtomentum. Ocellar setae well-
developed. Thorax grayish with pale golden microtomentum only laterally. Dorsocentral post-
sutural setae with 2 well differentiated and 1–3 anterior setae smaller. Apical scutellar seta
absent. Legs blackish. Abdomen with silvery microtomentum. T3 with 1 lateral marginal seta.
T4 with 1 median marginal and 1 lateral marginal setae. T5 with golden microtomentum in
part. ST5 with parallel cleft edges and with setae scattered on arm‟s surface. Cerci, in lateral
view, sinuous with pointed apexes of oblique upwards edges. Setae ventrally on the cerci

100
(lateral view) absent only in the middle portion. Apical shape of the cerci (last ⅓ portion of the
cercus; in posterior view) smaller than the middle part. Conformation of the cerci in posterior
view is parallel. Presence of a remarkable constriction at middle portion of the cerci, in
posterior view. In lateral view, distiphallus with presence of a ventroapical concavity with
smooth margins; distiphallic apical shape rounded, sinuous distiphallic dorsal shape. Presence
of lateroapical expansions on distiphallus in dorsal view. Pregonite with expanded base
narrowing smoothly until the apex, which is darker than the base, as well as the postgonite,
except for the color which is the same along the entire extension. Vesica with symmetrical
branches; terminal lobes reduced, with rounded shape and membranous texture; without
spines; and with presence of an angular median projection of the main vesica branch.
Remarks. A good and detailed male terminalia comparison of the sympatric species
not uncommonly misidentified, O. avuncula, O. confusa, O. diana and O. parva, is given by
Silva & Mello-Patiu (2008). Female was classified by Tibana & Mello (1985), according to
the shape of the T6+7, as syntergite undivided. See also “remarks” section on O. comparilis,
O. edwardsi and O. meridionalis for further discussion.
Distribution. NEOTROPICAL. Argentina (Jujuy, Misiones), Brazil (Ceará, Mato
Grosso, Minas Gerais, Rio de Janeiro, Paraná, São Paulo).
Biology. This species has been reared on human feces (Lopes 1973b; D‟Almeida
1994). Agar plus powder milk has been used successfully as artificial rearing source for O.
parva under laboratory conditions (Lopes 1973b). In our laboratory, it was reared on minced
bovine meat with larval stage during 6–7 days and pupal stage, until adult emergence, taking
9–11 days. Attractiveness has been already recorded by human feces, chicken viscera, fish,
mouse and pig carcasses, banana plus brown sugar and rotten Syagrus comosa (Mart.) Mart.
(Arecaceae), a species of a palmtree (at costal area) (Lopes 1973b; Lopes 1975a; Dias et al.
1984a; Barbosa et al. 2009; Vairo et al. 2011). Dias et al. (1984a) collected this species in
urban, rural and forest areas, although higher occurrence was observed only for the last
environment, considering O. parva as an asynanthropic species. It was also observed higher
preference for human feces comparing to the other substrates (chicken viscera, raw fish, mice
carcass and banana plus brown sugar) analyzed (Dias et al. 1984c).

101
Material examined. ♂: BRAZIL: São Paulo, Campinas, Sousas, 13.IV.2011, C. M.
Souza, D. L. Brancoli, F. Rezende / ??? 4 Sousas, Campinas-SP, 13/04/2011 / Oxy sp 1 (L2B-
DBA).
Oxysarcodexia paulistanensis (Mattos, 1919)
Sarcophaga paulistanensis Mattos, 1919
Oxysarcodexia amarali Prado & Fonseca, 1932
Oxysarcodexia delpontei Blanchard, 1939
Oxysarcodexia artigasi Dodge, 1966
Oxysarcodexia artegasi: Lopes (1973), incorrect subsequent spelling of artigasi
Dodge, 1966
(Appendix 25-B)
Type locality. Brazil, São Paulo. Mattos, 1919: 72.
Depository of type material. MZUSP.
Diagnosis. Male. Postocular plate with golden microtomentum. Ocellar setae not well-
developed. Thorax and abdomen with silvery microtomentum. Dorsocentral post-sutural setae
with 2 well differentiated and 1–3 anterior setae smaller. Apical scutellar seta present. Legs
blackish. T3 with 2 lateral marginal setae. T4 with 1 median marginal and 2 lateral marginal
setae. T5 without golden microtomentum. ST5 with parallel cleft edges and with setosity.
Cerci, in lateral view, sinuous with expanded apexes of oblique upwards edges. Setae ventrally
on the cerci (lateral view) present in full extension. Apical shape of the cerci (last ⅓ portion of
the cercus; in posterior view) smaller than the middle part. Conformation of the cerci in
posterior view is divergent. In lateral view, distiphallus with ventroapical concavity with
serrated margins; distiphallic apical shape rounded, sinuous distiphallic dorsal shape; and a
small distiphallic dorsoapical swelling. Pregonite and postgonite with expanded base
narrowing smoothly until the apex and same color along the entire extension. Vesica with
symmetrical branches; terminal lobes well-developed, filamentous at most tapering to apex
shape, sclerotized texture and with presence of lateral lobes (i.e. division of the vesica coming
from or close to the basal branch of the vesica, placed laterally to phallic tube); presence of

102
spines only on ventral surface; and presence of a rounded median projection of the main
vesica branch.
Remarks. See “remarks” section on O. bikini, O. injuncta and O. marina. Female was
classified by Tibana & Mello (1985), according to the shape of the T6+7, as syntergite
partially divided into two plates. Larval morphology of 1st, 2
nd and 3
rd instars was described by
Lopes (1943) and studied ultrastructurally, using scanning electron microscope, by Lopes &
Leite (1987).
Distribution. NEOTROPICAL. Argentina (Buenos Aires, Córdoba, Entre Ríos),
Brazil (Distrito Federal, Goiás, Minas Gerais, Paraná, Rio de Janeiro, Rio Grande do Sul,
Santa Catarina, São Paulo), Chile (Santiago).
Biology. Oxysarcodexia paulistanensis has been reared under laboratory conditions on
agar plus powder milk for 24h, then transferred to meat; and under natural conditions, on
human feces – which is the preferred substrate for rearing (Lopes 1973b; Mendes & Linhares
1993) –, rat carcas (Mendes & Linhares 1993; Moura 2004; Moura et al. 2005) and chicken
viscera (Mendes & Linhares 1993). Time of development reported in the literature for this fly
is 8 days of larval stage and 6–9 days in pupa, until the emergence of adults (Lopes 1943). At
our laboratory, it was reared on minced bovine meat, although larviposition was observed also
on rotten fish, with larval stage during 5–7 days and pupal stage, until adult emergence, taking
8–12 days. Baits as human and dog feces, chicken viscera, rotten bovine liver, fish, mouse, rat
and pig carcasses and banana plus brown sugar have been reported as attractive for this
species (Lopes 1973b; Ferreira et al. 1980; Dias et al. 1984c; Mendes & Linhares 1993;
Carvalho & Linhares 2001; Moura et al. 2005; Barros et al. 2008; Rosa et al. 2011; Vairo et
al. 2011; Beuter et al. 2012). In a study of synanthropy of flesh flies, Linhares (1981)
observed higher attraction of O. paulistanensis by human feces and mouse carcasses than to
chicken viscera, and also pointed out this species preference for sunlight instead of shaded
areas. The higher frequency of adult females on chicken viscera and rodent carcasses baits
suggested the use of these substrates as proteic resources for the development of ovarian
folicules (Mendes & Linhares 1993). Oxysarcodexia paulistanensis occurring at the coastline
of Buenos Aires was analysed taking into account its seasonal trends in abundance, habitat and
bait attractiveness, evidencing this species positive correlation with mean temperature,
preference by grassland instead of woodland and by dog feces instead of bovine liver (Mulieri

103
et al. 2008). This fly has been found both in urban, suburban and rural areas (Mulieri et al.
2011; Beuter et al. 2012). It is also considered a flower visitor of Coriandrum sativum L.
(Apiaceae), known as coriander or cilantro, and of Condalia spp., Discaria Americana Gillies
& Hook, Scutia buxifolia Reissek, shrubs of Rhamnaceae family (Mulieri et al. 2010).
Material examined. ♂♂: BRAZIL: São Paulo, Campinas, Sousas, 13.IV.2011, C. M.
Souza, D. L. Brancoli, F. Rezende / O. paulistanensis, Sousas, Campinas-SP, 13/04/2011
(L2B-DBA) // BRAZIL: São Paulo, Campinas, UNICAMP, IX.2010, C. M. Souza / O.
paulistanensis, Campinas-SP, SET/2010 (L2B-DBA).
Oxysarcodexia peculiaris Lopes, 1975
(Appendix 25-C)
Type locality. Brazil, Espírito Santo Linhares. Lopes, 1975c: 477.
Depository of type material. MNRJ.
Diagnosis. Male. Postocular plate with golden microtomentum. Ocellar setae well-
developed. Thorax with pale golden microtomentum. Dorsocentral post-sutural setae with 2
well differentiated and 2 anterior setae smaller. Apical scutellar seta present. Legs blackish.
Abdomen with silvery microtomentum, although some shades of pale golden microtomentum
is seen especially on T4 and T5. T3 with 2 lateral marginal setae. T4 with 1 median marginal
and 3 lateral marginal setae. T5 with golden microtomentum in part. ST5 with parallel cleft
edges and with setae on the apex of the arms. Cerci, in lateral view, sinuous with expanded
apexes of oblique upwards edges. Setae ventrally on the cerci (lateral view) present in apical
⅓ portion. Apical shape of the cerci (last ⅓ portion of the cercus; in posterior view) smaller
than the middle part. Conformation of the cerci in posterior view is divergent. In lateral view,
distiphallus with ventroapical concavity with smooth margins ventroapically; presence of
ventroapical projections; distiphallic apical shape conic and sinuous distiphallic dorsal shape.
Presence of a notable dilatation on the distiphallus lateroapically and membranous dilatations
medially on the phallus. Pregonite with expanded base narrowing smoothly until the apex and
same color along the entire extension. Postgonite with expanded base and sudden narrowing at
apex and same color along the entire extension. Vesica with symmetrical branches; terminal
lobes well-developed, with rounded shape and sclerotized texture; presence of spines only on
ventral surface; and presence of a rounded median projection of the main vesica branch.

104
Remarks. See “remarks” section on O. jamesi.
Distribution. NEOTROPICAL. Brazil (Espírito Santo, Rio de Janeiro).
Biology. The paratype species was reared from human feces (Lopes 1975c), however
without mention any further information about its development.
Material examined. ♂: Linhares, E. Santo, Brasil / P. C. Elias VI-72 / Holotype /
Oxysarcodexia peculiaris ♂ n.sp. Det. H. S. Lopes / MNRJ/2249 (MNRJ).
Oxysarcodexia peltata (Aldrich, 1916)
Sarcophaga peltata Aldrich, 1916
(Appendix 26-A)
Type locality. Puerto Rico, Mayaguez. Aldrich, 1916: 216.
Depository of type material. USNM.
Diagnosis. Male. Postocular plate with golden microtomentum. Ocellar setae well-
developed. Thorax and abdomen with golden microtomentum. Dorsocentral post-sutural setae
with 2 well differentiated and 1–3 anterior setae smaller. Apical scutellar seta absent. Legs
brownish. T3 with 1 lateral marginal seta. T4 without median marginal and with 1 lateral
marginal seta. T5 with golden microtomentum in part. ST5 with parallel cleft edges and with
setae scattered on the apex of the arms. Cerci, in lateral view, bent backwards with normal
apexes (same size as the median area) of concave edges. Setae ventrally on the cerci (lateral
view) present on apical ⅓ portion. Apical shape of the cerci (last ⅓ portion of the cercus; in
posterior view) smaller than the middle part. Conformation of the cerci in posterior view is
divergent. In lateral view, distiphallus with smooth margins ventroapically; distiphallic apical
conic shape, straight distiphallic dorsal shape; presence of a large dorsoapical swelling and
lateral lobes on distiphallus. Pregonite with expanded base and sudden narrowing at apex,
which is darker than the base. Postgonite with expanded base narrowing smoothly until the
apex and same color along the entire extension. Vesica with symmetrical branches; terminal
lobes reduced, with filamentous at most tapering to apex shape and partially membranous
texture; presence of spines on both dorsal and ventral surfaces; and presence of an angular
median projection of the main vesica branch.
Remarks. See “remarks” section on O. aurata. Female was classified by Tibana &
Mello (1985), according to the shape of the T6+7, as syntergite membranous.

105
Distribution. NEARTIC. USA (Florida). NEOTROPICAL. Bahamas (Andros, Cat I,
Eleuthera, Grand Bahama, Great Inagua, New Providence), Cuba (Havana), Dominica,
Guadalupe, Jamaica, Mexico (Jalisco, Tabasco), Panama, Puerto Rico, San Andrés Islands,
Santa Lucia, San Vicente.
Biology. Despite of the type-species status for the genus Oxysarcodexia, the only
information about O. peltata biology is the relationship with Laguncularia racemosa (L.) c.f.
Gaertn (Combretaceae), being a probable highly effective pollinator of this mangrove species
(Sánchez-Núñez & Mancera-Pineda 2012).
Material examined. ♂: Est. Cent. Agr. De Cuba 8089 / Sarcophaga peltata Ald. / R.
Panke/1/9/1920 (ZMUC).
Oxysarcodexia perneta (Walker, 1861)
Sarcophaga perneta Walker, 1861
(Appendix 26-B)
Type locality. Mexico. Walker, 1861: 308.
Depository of type material. BMNH.
Diagnosis. Male. Postocular plate with golden microtomentum. Ocellar setae not well-
developed. Thorax and abdomen with golden microtomentum. Dorsocentral post-sutural setae
with 2 well differentiated and 1–3 anterior setae smaller. Apical scutellar seta present. Legs
brownish. T3 with 1 lateral marginal seta. T4 with 1 median marginal and 2 lateral marginal
setae. T5 with golden microtomentum along the entire extension. ST5 with parallel cleft edges
and with setosity and setae on the apex of the arms. Cerci, in lateral view, straight with pointed
apexes of oblique upwards edges. Setae ventrally on the cerci (lateral view) present in full
extension. Apical shape of the cerci (last ⅓ portion of the cercus; in posterior view) smaller
than the middle part. Conformation of the cerci in posterior view is parallel. Presence of a
remarkable constriction at middle portion of the cerci, in posterior view. In lateral view,
distiphallus with presence of a ventroapical concavity of smooth margins; distiphallic apical
rounded shape; straight distiphallic dorsal shape; and presence of a small dorsoapical swelling
on distiphallus. Pregonite and postgonite with expanded base narrowing smoothly until the
apex and same color along the entire extension. Vesica with symmetrical branches; terminal
lobes well-developed, with rounded shape and membranous texture; presence of spines on

106
both dorsal and ventral surfaces; and presence of a rounded median projection of the main
vesica branch.
Remarks. Lopes (1946b) indicated differences on vesica appearance due to specimen
preparation. In dry pinned flies, it can seem juxtaposed to the distiphallus, whereas in wet
preparation or dried properly, the vesica become detached from distiphallus and examination
of distiphallus apical shape and vesica terminal lobes can be easier done. See “remarks”
section of Oxysarcodexia n. sp. 3.
Distribution. NEARTIC. Mexico (Districto Federal, Morelos). NEOTROPICAL.
Honduras, Mexico (Oxaca).
Biology. Unknown. Information available is only about the type of collection, active,
using an entomological net, or with traps for collecting fruit flies (Lopes 1946c).
Material examined. ♂♂: MEX. Chiapas, San Cristobal 7000m, 4 May 1969, H. J.
Teskey / Oxysarc.perneta (Wlk.), det. Lopes (ZMUC) // Col. Inst. O. Cruz N. 9.361 / Mexico
D.F., A. Dampf [unreadable number] / Oxysarcodexia perneta Wulp ♂ xi-44 Det. H. S. Lopes
(MNRJ) // Cuernavaca Est. Morelos Mexico 1800m Dampf 1.IX a 5.XII / Oxysarcodexia
perneta (Walker) Det. H. S. Lopes ♂ (MNRJ).
Oxysarcodexia peruviana (Lopes, 1975)
Xarcophaga titubata ssp. peruviana Lopes, 1975
(Appendix 26-C)
Type locality. Peru, Lambayeque, La Beatita. Lopes, 1975f: 575.
Depository of type material. UNPRG.
Diagnosis. Male. Postocular plate with intense golden microtomentum. Ocellar setae
well-developed. Thorax gray with golden microtomentum more intense on humeral region.
Dorsocentral post-sutural setae with 3 well-differentiated setae. Presence of apical scutellar
seta, small though. Abdomen with golden microtomentum more intense laterally on T4 and
T5. T4 without median marginal setae. ST5 reddish. Cerci curved backwards (lateral view).
Phallus similar to the typical form, but apical and lateral plates besides vesica with different
shapes. This is a synopsis of original description given by Lopes (1975f).
Remarks. See “remarks” section on Oxysarcodexia n. sp. 4, O. augusta and O.
fraterna.

107
Distribution. NEOTROPICAL. Colombia (Antioquia), Peru (Lambayeque).
Biology. Unknown.
Oxysarcodexia petropolitana Lopes, 1975
(Appendix 27-A)
Type locality. BRAZIL: Rio de Janeiro. Lopes, 1975c: 471.
Depository of type material. MNRJ.
Diagnosis. Male. Postocular plate with golden microtomentum. Ocellar setae not well-
developed. Thorax with golden microtomentum. Dorsocentral post-sutural setae with 2 well
differentiated and 3 anterior setae smaller. Apical scutellar seta absent. Legs brownish.
Abdomen with silvery microtomentum, although some shades of pale golden microtomentum
is seen laterally. T3 with 2 lateral marginal setae. T4 with 1 median marginal and 2 lateral
marginal setae. T5 with golden microtomentum in part. ST5 with parallel cleft edges,
triangular apical shape of the arms and with setosity scattered on arms‟ surface. Cerci, in
lateral view, straight with normal apexes (same size as the median area) of concave edges.
Setae ventrally on the cerci (lateral view) present in apical ⅓ portion. Apical shape of the cerci
(last ⅓ portion of the cercus; in posterior view) with the same size as the middle part.
Conformation of the cerci in posterior view is parallel. Presence of a remarkable constriction
at middle portion of the cerci, in posterior view. In lateral view, distiphallus with smooth
margins ventroapically; distiphallic apical shape rounded and sinuous distiphallic dorsal
shape. Pregonite with a mediobasal projection (“thorn-like”) and expanded base narrowing
smoothly until the apex and same color along the entire extension. Postgonite with expanded
base and sudden narrowing at apex and same color along the entire extension. Vesica with
symmetrical branches; presence of lateral lobes (i.e. division of the vesica coming from or
close to the basal branch of the vesica, placed laterally to phallic tube); terminal lobes well-
developed, with sclerotized texture, filamentous at most tapering to apex shape, with basal
portion enlarged and apexes pointed backwards; presence of spines only on ventral surface;
and presence of a rounded median projection of the main vesica branch.
Remarks. See “remarks” section on O. insolita. Female was described by Lopes
(1975c) with golden microtomentum on T6+7, which is composed by two plates; sternites

108
reddish and anal tergite represented by a pair of setae. It was classified by Tibana & Mello
(1985), according to the shape of the T6+7, as syntergite partially divided into two plates.
Biology. Species already reared on human feces and on meat (Lopes 1973b; Lopes
1975c), although no other details of its development are reported. Attractiveness is reported
only for human feces (Lopes 1973b).
Distribution. NEOTROPICAL. Brazil (Minas Gerais, Rio de Janeiro, Santa Catarina,
São Paulo), Ecuador.
Material examined. ♂: 6 / Estr. S. Paulo – Santos, Brasil 3.II.68 R. Kano / Paratype /
Oxysarcodexia petropolitana Lopes H. S. Lopes det. (MNRJ).
Oxysarcodexia plebeja Lopes, 1946
(Appendix 27-B)
Type locality. Mexico, Morelos, Cuernavaca. Lopes, 1946c: 142.
Depository of type material. MNRJ.
Diagnosis. Male. Postocular plate with golden microtomentum. Ocellar setae not well-
developed. Thorax with golden microtomentum more intense laterally. Dorsocentral post-
sutural setae with 3 well differentiated setae, although a small seta among these 3 can be
present. Apical scutellar seta present. Legs blackish. Abdomen with intense golden
microtomentum. T4 with 2 lateral marginal setae. T5 with golden microtomentum along the
entire extension. ST5 with parallel cleft edges and with setosity and setae on the apex of the
arms. Cerci, in lateral view, straight with expanded apexes of oblique upwards edges. Setae
ventrally on the cerci (lateral view) present on apical half. Apical shape of the cerci (last ⅓
portion of the cercus; in posterior view) smaller than the middle part. Conformation of the
cerci in posterior view is parallel. In lateral view, distiphallus with presence of a ventroapical
concavity of serrated margins; distiphallic apical rounded shape; straight distiphallic dorsal
shape. Pregonite and postgonite with expanded base narrowing smoothly until the apex and
same color along the entire extension. Vesica with symmetrical branches; terminal lobes
reduced, with lateral expansions of filamentous at most tapering to apex shape and sclerotized
texture; presence of spines only on ventral surface of the median lobe; and presence of a
rounded median projection of the main vesica branch.

109
Remarks. Vesica shape is very peculiar and different from the typical shape seen into
the genus. See also “remarks” section on O. augusta.
Distribution. NEARTIC. Mexico (Morelos, San Luis Potosí), USA (Texas).
NEOTROPICAL. Colombia (Antioquia), Costa Rica, Mexico (Chiapas).
Biology. Original description gives information only about the type of collection, using
traps for collecting fruit flies, without further specification (Lopes 1946c). At Antioquia,
Colombia, this species was collected using chicken viscera and fish head as attractive baits
(Ramírez-Mora et al. 2012).
Material examined. ♂♂: SP 25 / Pajar. Mar 12 / O. plebeja ? (CE-TdeA) [from
Colombia] // MEX. Chis. 32mi W. San Cristobal Jct 190–195 Hwys. 12, V, 1969 H. J. Teskey
/ Oxysarc. plebeja ♂ Lopes Det. H. S. Lopes (MNRJ).
Oxysarcodexia ramosa (Reinhard, 1939)
Sarcophaga ramosa Reinhard, 1939
(Appendix 27-C)
Type locality. USA, Texas, Donnas. Reinhard, 1939: 64.
Depository of type material. Unknown.
Diagnosis. Male. Head yellowish-gray. Ocellar setae not well-developed. Thorax
grayish. Four dorsocentral post-sutural setae. Apical scutellar seta is absent. Legs blackish.
Abdomen yellowish-gray. T5 reddish. ST5 reddish and with a U-shaped cleft. Phallus with
vesica of asymmetrical branches; presence of flattened squamas on the smaller branch. This is
a synopsis of diagnoses given by Lopes (1946b).
Remarks. Oxysarcodexia ramosa is one of the species of this genus that have a
noteworthy asymmetrical vesica.
Distribution. NEARTIC. USA (Texas).
Biology. Unknown.
Oxysarcodexia riograndensis Lopes, 1946
(Appendix 28-A)
Type locality. Brazil, Rio Grande do Sul, São João de Montenegro. Lopes, 1946b:
103.

110
Depository of type material. MNRJ.
Diagnosis. Male. Postocular plate with golden microtomentum. Ocellar setae well-
developed. Thorax and abdomen with silvery microtomentum. Dorsocentral post-sutural setae
with 2 well differentiated and 1–3 anterior setae smaller. Apical scutellar seta present. Legs
blackish. T3 with 2 lateral marginal setae. T4 with 1 median marginal and 2 lateral marginal
setae. T5 without golden microtomentum. ST5 with parallel cleft edges and with setosity.
Cerci, in lateral view, straight with expanded apexes of oblique upwards edges. Setae ventrally
on the cerci (lateral view) absent only on the middle portion. Apical shape of the cerci (last ⅓
portion of the cercus; in posterior view) smaller than the middle part. Conformation of the
cerci in posterior view is divergent. In lateral view, distiphallus with ventroapical concavity of
serrated margins; distiphallic apical square/oblong shape, sinuous distiphallic dorsal shape;
presence of a medium dorsoapical swelling on distiphallus. Pregonite and postgonite with
expanded base narrowing smoothly until the apex and same color along the entire extension.
Vesica with symmetrical branches; presence of lateral lobes (i.e. division of the vesica coming
from or close to the basal branch of the vesica, placed laterally to phallic tube); terminal lobes
well-developed, with rounded shape, sclerotized texture and spines present only on ventral
surface; and presence of a rounded median projection of the main vesica branch.
Remarks. See “remarks” section on O. bikini. Female was classified by Tibana &
Mello (1985), according to the shape of the T6+7, as syntergite undivided.
Distribution. NEOTROPICAL. Argentina (Jujuy), Brazil (Minas Gerais, Paraná,
Pernambuco, Rio de Janeiro, Rio Grande do Sul, São Paulo).
Biology. This species has been reared from a corpse on bloated stage and collected on
the premises of an institute of legal medicine using chicken liver as bait and, for this,
considered as necrophagous/omnivore species (Oliveira & Vasconcelos 2010). At our
laboratory, it was reared only once, on minced bovine meat with larval stage during 6 days and
pupal stage, until adult emergence, taking 9 days. In a study comparing the attractiveness of
baits, Linhares (1981) observed that human feces and mouse carcasses were more attractive
than chicken viscera for O. riograndensis, pattern corroborated by Mendes & Linhares (1993).
Sunlight instead of shaded areas is also referred as a preference of this species (Linhares
1981). This fly has also been collected using mice, rat, rabbit and pig carcasses, fish, chicken
liver and human feces as baits (Ferreira 1979; Mendes & Linhares 1993; Carvalho & Linhares

111
2001; Cruz 2008; Moretti et al. 2008; Rosa et al. 2011; Vairo et al. 2011; Vasconcelos &
Araújo 2012). Adult specimens have been found both in urban, rural or forest environments
(Linhares 1981; Mendes & Linhares 1993; Rosa et al. 2011; Vairo et al. 2011; Vasconcelos &
Araújo 2012).
Material examined. ♂: BRAZIL: São Paulo, Campinas, UNICAMP, 18.VII.2010, C.
M. Souza / O. riograndensis, Campinas-SP, 18/08/2010 (L2B-DBA).
Oxysarcodexia sarcinata Lopes, 1953
(Appendix 28-B)
Type locality. Colombia, Medellín. Lopes, 1953: 48.
Depository of type material. AMNH.
Diagnosis. Male. Postocular plate with golden microtomentum. Ocellar setae well-
developed. Thorax and abdomen with golden microtomentum. Dorsocentral post-sutural setae
with 2 well differentiated and 1–3 anterior setae smaller. Apical scutellar seta present. Legs
blackish. T3 with 2 lateral marginal setae. T4 with 1 median marginal and with 2 lateral
marginal setae. T5 with golden microtomentum in part. ST5 with parallel cleft edges and with
setae on the apical half of the arms. Cerci, in lateral view, sinuous with expanded apexes of
oblique upwards edges. Setae ventrally on the cerci (lateral view) absent only on the middle
portion. Apical shape of the cerci (last ⅓ portion of the cercus; in posterior view) bigger than
the middle part. Conformation of the cerci in posterior view is divergent. Presence of a
remarkable constriction at middle portion of the cerci, in posterior view. In lateral view,
presence of a ventroapical concavity on distiphallus with smooth margins; distiphallic apical
rounded shape and sinuous distiphallic dorsal shape. Pregonite with expanded base and
sudden narrowing at apex and same color along the entire extension. Postgonite with expanded
base narrowing smoothly until the apex. Vesica with notably asymmetrical branches; terminal
lobes well-developed, with filamentous at most tapering to apex shape and sclerotized texture;
presence of spines only on ventral surface; and presence of an angular median projection of
the main vesica branch.
Remarks. Oxysarcodexia sarcinata is one of the species of this genus that have a
noteworthy asymmetrical vesica.

112
Biology. This fly specimen has already been collected from over a swarm raid of
Eciton burchelli (Westwood, 1842), an ant species (Dodge 1968). Chicken viscera and fish
head were baits used to attract and collect them in Antioquia, Colombia (Ramírez-Mora et al.
2012).
Distribution. NEARTIC. Mexico (San Luis Potosí). NEOTROPICAL. Colombia
(Antioquia, Calí, Medellín), Costa Rica, Mexico (Alajuela, Chiapas, Guanacaste, Heredia),
Panama (Barro Colorado Island), Trinidad and Tobago (Trinidad).
Material examined. ♂♂: PANAMA: Veraguas Pr. Calovébora, Rio Guazarito 14–19
Feb 2000 Col. S. Bermudez C. / 54 / NRM-DIPT 0014326 (NRM) // COLOMBIA: Antioquia,
Caldas, La Clara, 06°03‟06,9”N 75°37‟19,2”W 1.640m 7.ii.2010 Tr VSR 15:22 Cols. M. A.
Ramírez, J. Durango, H. Areiza / Dexosarcophaga, Det. M. A. Ramírez / Oxysarcodexia
sarcinata (CE-TdeA) // COSTA RICA: Alajuela prov. Atenas-Orotina road, Cerro del
Aguacate, 869m, 14.viii.2010, T. Pape, 9°58‟25,12”N 84°24‟52,24”W (ZMUC) // Costa Rica:
Cartago Turialba 2000 23 July 1965 H. .G Real / Oxysarc. sarcinata ♂ Lop. Det. H. S. Lopes
(MNRJ).
Oxysarcodexia similata Lopes & Tibana, 1987
(Appendix 28-C)
Type locality. Trinidad and Tobago, Trinidad, Aripo. Lopes & Tibana, 1987: 335.
Depository of type material. MNRJ.
Diagnosis. Male. Postocular plate with pale golden microtomentum. Ocellar setae
well-developed. Thorax and abdomen with not very intense golden microtomentum.
Dorsocentral post-sutural setae with 2 well differentiated and 1–3 anterior setae smaller.
Apical scutellar seta present. Legs blackish. T3 with 2 lateral marginal setae. T4 with 1
median marginal and with 1 lateral marginal seta. T5 with golden microtomentum in part. ST5
with parallel cleft edges and with setosity. Cerci, in lateral view, straight with expanded
apexes of oblique upwards edges. Setae ventrally on the cerci (lateral view) present in full
extension. Apical shape of the cerci (last ⅓ portion of the cercus; in posterior view) smaller
than the middle part. Conformation of the cerci in posterior view is divergent. In lateral view,
distiphallus with smooth margins ventroapically; distiphallic apical rounded shape and straight
distiphallic dorsal shape. Pregonite with expanded base narrowing smoothly until the apex and

113
same color along the entire extension. Postgonite with expanded base and sudden narrowing at
apex. Vesica with symmetrical branches; presence of lateral lobes (i.e. division of the vesica
coming from or close to the basal branch of the vesica, placed laterally to phallic tube),
terminal lobes well-developed, with filamentous at most tapering to apex shape and
sclerotized texture; presence of spines only on ventral surface; and presence of a rounded
median projection of the main vesica branch.
Remarks. Oxysarcodexia similata can be misidentified as O. amorosa and O.
xanthosoma due to morphological similarities seen on the male terminalia. See “remarks”
section on Oxysarcodexia n. sp. 1 and O. amorosa for further discussion. Female was
described together with the male; it presents a blackish membrane between T5 and T6+7,
golden microtomentum between plates of genital tergite and red genital sternites (Lopes &
Tibana 1987).
Distribution. NEARTIC. Mexico (Morelos, San Luis Potosí). NEOTROPICAL.
Colombia (Antioquia), Costa Rica, Guyana, Mexico (Jalisco), Panama, Trinidad and Tobago
(Trinidad).
Biology. Oxysarcodexia similata showed strong attractiveness for chicken viscera
despite of human feces, which attracted no specimens. In Antioquia, Colombia, occurrence of
O. similata was reported for urban and rural areas and only a low abundance in the forest
environment, pointing out the preference of this species for human settlements (Yepes-
Gaurisas et al. 2013).
Material examined. ♂♂: PANAMA: Panama prov. Punta Charme, 19.ix.2002, J.
Méndez 79°45‟W 8°40‟N red mangrove stand / NRM-DIPT 0014339 (NRM) // Costa Rica:
Cartago Turrialba 2000‟ 23 July 1965 H. G. Real / Herman G. Real Collection / Paratype /
Oxysarcodexia similata n.sp. ♂ Paratipo Tibana & Det. H. S. Lopes.
Oxysarcodexia simplicoides (Lopes, 1933)
Sarcophaga simplicoides Lopes, 1933
(Appendix 29-A)
Type locality. Brazil, Rio de Janeiro, Guanabara. Lopes, 1933: 156.
Depository of type material. MNRJ.

114
Diagnosis. Male. Postocular plate with silvery microtomentum. Ocellar setae well-
developed. Thorax and abdomen with silvery microtomentum. Dorsocentral post-sutural setae
with 2 well differentiated and 3 anterior setae smaller. Apical scutellar seta absent. Legs
brownish. T3 with 3 lateral marginal setae. T4 with 1 median marginal and 2 lateral marginal
setae. T5 without golden microtomentum, only silvery microtomentum is seen. ST5 with
parallel cleft edges and with setosity. Cerci, in lateral view, sinuous with pointed apexes of
straight edges. Setae ventrally on the cerci (lateral view) present in full extension. Apical
shape of the cerci (last ⅓ portion of the cercus; in posterior view) with the same size as the
middle part. Conformation of the cerci in posterior view is divergent. Presence of a remarkable
constriction at middle portion of the cerci, in posterior view. In lateral view, distiphallus with
smooth margins ventroapically and presence of ventroapical projections; distiphallic apical
shape rounded and straight distiphallic dorsal shape. Presence of a notable dilatation on the
distiphallus lateroapically. Pregonite and postgonite with expanded base narrowing smoothly
until the apex and same color along the entire extension. Vesica with symmetrical branches;
presence of lateral lobes (i.e. division of the vesica coming from or close to the basal branch of
the vesica, placed laterally to phallic tube); terminal lobes well-developed, with rounded
shape, sclerotized texture and without spines; basal branch enlarged.
Remarks. This fly is considered a rare species. The presence of slender setulae on the
gena and the postocullar plate with same color as the occiput are its more peculiar
characteristics (Lopes 1933). Female was classified by Tibana & Mello (1985), according to
the shape of the T6+7, as syntergite partially divided into two plates.
Distribution. NEOTROPICAL. Brazil (Ceará, Mato Grosso, Mato Grosso do Sul,
Minas Gerais, Rio de Janeiro).
Biology. Information is concerned about collection methods and attractiveness. Baits
as banana plus brown sugar, cow liver and lung, fish and squid has been used with traps
(“can” traps), whereas feces has been an attractive substrate for collecting actively (Lopes
1975a; Pamplona et al. 2000). Adults of O. simplicoides have also been associated to pig and
rodents carcasses in urban and natural areas of Brazilian Cerrado (“savanna-like”) vegetation
(Barbosa et al. 2009; Rosa et al. 2011; Vasconcelos & Araújo 2012).
Material examined. ♂: P. C. Elias VI-72 / Linhares, E. Santo, Brasil / simplicoides
(MNRJ).

115
Oxysarcodexia terminalis (Wiedemann, 1830)
Sarcophaga terminalis Wiedemann, 1830
Amesothyrsus chilensis Enderlein, 1928
Sarcophaga complicata Hall, 1937
Hybopygia pseudovaria Blanchard, 1939
(Appendix 29-B)
Type locality. Brazil. Wiedemann, 1830: 366.
Depository of type material. Unknown.
Diagnosis. Male. Postocular plate with silveryish microtomentum. Ocellar setae well-
developed. Thorax and abdomen with golden microtomentum more evident laterally.
Dorsocentral post-sutural setae with 3 well differentiated setae, although a small seta among
these 3 can be present. Apical scutellar seta absent. Legs brownish. T3 with 2 lateral marginal
setae. T4 with 1 median marginal and 1 lateral marginal setae. T5 with golden microtomentum
in part. ST5 with V-shaped cleft edges and setosity and setae along the edges of the arms.
Cerci, in lateral view, bent backwards with pointed apexes of oblique upwards edges. Setae
ventrally on the cerci (lateral view) present at apical ⅓ portion. Apical shape of the cerci (last
⅓ portion of the cercus; in posterior view) smaller than the middle part. Conformation of the
cerci in posterior view is divergent. In lateral view, distiphallus with smooth margins
ventroapically; distiphallic apical square/oblong shape and sinuous distiphallic dorsal shape.
Pregonite with base and apex with same size and same color along the entire extension.
Postgonite with expanded base narrowing smoothly until the apex. Vesica with symmetrical
branches; presence of lateral lobes (i.e. division of the vesica coming from or close to the basal
branch of the vesica, placed laterally to phallic tube); terminal lobes well-developed, with
filamentous at most tapering to apex shape, partially membranous texture, and presence of
spines only along the edges.
Remarks. Cerci bent backwards (lateral view) and with slender shape (dorsal view)
associated to the apical distiphallus shape and explanated vesica enable the easy recognition of

116
this species. See also “remarks” section on O. augusta. Female presents genital segments
retracted, reddish and with golden microtomentum (Blanchard 1939).
Distribution. NEOTROPICAL. Argentina (Buenos Aires, Córdoba, La Rioja), Brazil
(Goiás, Minas Gerais, Paraná, Rio de Janeiro, São Paulo), Chile (Coquimbo, Nuble,
Patagonia), Easter Islands, Peru.
Biology. Oxysarcodexia terminalis is listed as a coprophagous species, reared in cattle
dung and shed (Marchiori & Linhares 1999; Marchiori 2000; Marchiori et al. 2001; Mendes &
Linhares 2002; Mulieri et al. 2010). This species has already been reared under laboratory
conditions (Lopes 1973b), although no further information about its development was
documented. Besides bovine, dog and human feces, which are the preferential baits (Ferreira
1979; Linhares 1981; Dias et al. 1984c; Mulieri et al. 2010), attractiveness has also been
observed by chicken viscera, bovine liver, mouse, rat and pig carcasses, fish, crab, fermented
grapes, rotten banana plus brown sugar and rotten Syagrus comosa (Mart.) Mart. (Arecaceae),
a species of a palmtree (at coastal area) (Lopes 1973b; Dias et al. 1984c; Flores & Dale 1995;
Mariluis et al. 2007; Mulieri et al. 2008; Mulieri et al. 2011; Rosa et al. 2011; Beuter et al.
2012). Sunlight instead of shaded areas are also referred as a preference of this species
(Linhares 1981). Adults of O. terminalis are also among the Oxysarcodexia species already
collected associated to cadavers (Oliveira-Costa et al. 2001). Another ecological role of this
fly is the association as flower visitor with shrubs species (Baccharis spp.) of Asteraceae
family (Mulieri et al. 2010). This fly has been collected on grassland, urban, suburban and
rural areas (Mulieri et al. 2008; Mulieri et al 2011; Beuter et al. 2012), showing synanthropic
habits and populational peaks from September to December (Linhares 1981; Dias et al. 1984b;
Mendes & Linhares 1993; Mulieri et al. 2010).
Material examined. ♂♂: Brasília, D.F., Brasil, XI-60, A. B. Guimarães / NRM-DIPT
0014357 (NRM) // On human feces / H. S. Lopes 16.V.85 / Retiro Petrópolis E. do Rio, Brasil
/ O. terminalis (MNRJ).
Oxysarcodexia thornax (Walker, 1849)
Musca auriflua Wiedemann, 1830. Unavailable name. [Originally proposed in
synonymy with Musca tessellata Fabricius, 1805 and not validated by subsequent usage.]
Sarcophaga thornax Walker, 1849

117
Sarcophaga pudica Rondani, 1850
Sarcophaga aurifinis Walker, 1853
Hybopygia auricauda Enderlein, 1928
Oxysarcodexia neotropicale Prado & Fonseca, 1932
(Appendix 29-C)
Type locality. Unknown. Walker, 1849: 814.
Depository of type material. BMNH.
Diagnosis. Male. Postocular plate with pale golden microtomentum. Ocellar setae not
well-developed. Thorax and abdomen with pale golden microtomentum. Dorsocentral post-
sutural setae with 2 well differentiated and 1–3 anterior setae smaller. Apical scutellar seta
absent. Legs blackish. T3 with 2 lateral marginal setae. T4 with 1 median marginal and 2
lateral marginal setae. T5 with golden microtomentum in part. ST5 with parallel cleft edges
and with setosity and setae on the apex of the arms. Cerci, in lateral view, straight with normal
(same size as median area) apexes of concave edges. Setae ventrally on the cerci (lateral view)
absent only on the middle portion. Apical shape of the cerci (last ⅓ portion of the cercus; in
posterior view) with the same size as the middle part. Conformation of the cerci in posterior
view is divergent. In lateral view, distiphallus with serrated margins ventroapically;
distiphallic apical rounded shape and straight dorsal shape; presence of a “finger-like”
swelling dorsoapically. Pregonite and postgonite with expanded base and sudden narrowing at
apex, which, only in pregonite, is darker than the base. Vesica with symmetrical branches;
terminal lobes well-developed, with square shape and sclerotized texture; presence of spines
only along the edges; and presence of an angular median projection of the main vesica branch.
Remarks. See “remarks” section on O. afficta, O. conclausa and O. morretesi. Female
was classified by Tibana & Mello (1985), according to the shape of the T6+7, as syntergite
partially divided into two plates. Larvae are characterized by the presence of sinuous ribbons
of festoon on psedocephalon and conspicuous teeth on maxillae, same as O. confusa, differing
from it by the teeth of the maxillae and the festoon arms (Leite & Lopes 1987; Lopes & Leite
1986; 1987). This species has been intercepted in imported container with cargoes from
Americas in Shanghai Port, China (Deng et al. 2011).
Distribution. NEOTROPICAL. Argentina (Bahía Blanca, Buenos Aires, Catamarca,
Corrientes, Jujuy, Misiones, Tucumán), Bolivia, Brazil (Amapá, Amazonas, Ceará, Distrito

118
Federal, Espírito Santo, Goiás, Mato Grosso, Mato Grosso do Sul, Minas Gerais, Pará,
Paraíba, Paraná, Pernambuco, Rio de Janeiro, Rio Grande do Sul, Roraima, Santa Catarina,
São Paulo), Colombia (Antioquia), Ecuador (Pastaza), Guyana, Paraguay, Peru. PALEARTIC.
China (Shangai).
Biology. Oxysarcodexia thornax is one of the most widespread Oxysarcodexia species.
It is very abundant among flesh flies collected, especially during summer and autumn in Brazil
(Oliveira et al. 2002; Mulieri et al. 2010). Human and bovine feces are the preferred substrates
of O. thornax for rearing and also for feeding, especially for females in order to complete
ovarian development (Mendes & Linhares 1993; Mendes & Linhares 2002). Larviposition is
documented for human, dog and anteater feces, dung, shrimp, bovine kidney, chicken viscera
and dead fish (Lopes 1973b; D‟Almeida 1986; 1988; 1989; 1994; Mendes & Linhares 1993;
Marchiori & Linhares 1999; Marchiori et al. 2001; Marchiori et al. 2002; Mendes & Linhares
2002; Mulieri et al. 2010). Under laboratory conditions, O. thornax has been reared on agar
plus powder milk for 24h, and then transferred to meat in order to complete the development
until the emergence of adults (Lopes 1973b). At our laboratory, it was reared on minced
bovine meat with adults emerging after 17–21 days (8–12 days from larva to pupa and 5–11
days until the emergence of adults). This species has already been collected associated to pig
carcass (Carvalho & Linhares 2001; Barros et al. 2008; Barbosa et al. 2009; Vairo et al.
2011). Besides these, baits as raw pork, mice, rat and rodent carcasses, chicken viscera
(especially liver), fresh and rotten bovine liver and lung, fish, crab, squid, fermented banana,
banana plus brown sugar, rotten Syagrus comosa (Mart.) Mart. (Arecaceae) (a species of a
palmtree – at coastal area) have been reported as attractive for collecting this fly (Lopes
1973b; Lopes 1975a; Ferreira 1979; Ferreira et al. 1980; Dias et al. 1984c; D‟Almeida &
Lima 1994; Couri et al. 2000; Pamplona et al. 2000; Oliveira et al. 2002; Leandro &
D‟Almeida 2005; Costamagna et al. 2007; Marchiori 2007; Mariluis et al. 2007; Moretti et al.
2008; Mulieri et al. 2008; Mulieri et al. 2011; Sousa et al. 2011; Beuter et al. 2012;
Vasconcelos & Araújo 2012; Ramírez-Mora et al. 2012; Moretti & Godoy 2013). Adults have
also been collected associated to cadavers (Oliveira-Costa et al. 2001). Sunlight instead of
shaded areas is also referred as a preference of this species (Linhares 1981). The presence of
O. thornax is known for urban areas, rural areas, pasture, cattle shed, slaughterhouse,
zoological garden, highlands (at 1,000m of altitude), grassland, woodland, archipelago,

119
deforestation area (Couri et al. 2000; Marchiori 2000; Costamagna et al. 2007; Marchiori et
al. 2007a; Mariluis et al. 2007; Couri et al. 2008; Mulieri et al. 2008; Mulieri et al. 2011;
Beuter et al. 2012; Vasconcelos & Araújo 2012). D‟Almeida (1984) pointed out O. thornax
synanthropy related to inhabited areas, which seems a pattern changed along the time, once the
presence of this fly is reported for a wide variety of sites. Other ecological interactions
reported for this species are its role as host of parasitoids such as Trybliographa sp.
(Hymenoptera: Figitidae) (Marchiori et al. 2005; Marchiori et al. 2007a; Marchiori et al.
2007b); as an eventual or rare flower visitor on Metrodorea stipularis Mart. (Rutaceae), a
riparian forest tree species (Pombal & Morellato 2000); and as host of the rove beetles
Aleochara verberans Erichson, 1839 and A. bimaculata Gravenhorst, 1802 (Coleoptera:
Staphilinidae) (Walsh & Posse 2003).
Material examined. ♂: BRAZIL: São Paulo, Campinas, Sousas, 13.IV.2011, C. M.
Souza, D. L. Brancoli, F. Rezende / O. thornax, Sousas, Campinas-SP, 13/04/2011 (L2B-
DBA).
Oxysarcodexia timida (Aldrich, 1916)
Sarcophaga timida Aldrich, 1916
Sarcophaga sanguisuga Hall, 1933
(Appendix 30-A)
Type locality. Guatemala, Porto Barrios. Aldrich, 1916: 283.
Depository of type material. USNM.
Diagnosis. Male. Postocular plate with golden microtomentum. Ocellar setae not well-
developed. Thorax and abdomen grayish sometimes with slight pale golden microtomentum.
Dorsocentral post-sutural setae with 2 well differentiated and 1–3 anterior setae smaller.
Apical scutellar seta absent. Legs blackish. T3 with 2 lateral marginal setae. T4 with 1 median
marginal and 1 lateral marginal seta. T5 with golden microtomentum in part. ST5 with parallel
cleft edges and with setosity and setae on the apex of the arms. Cerci, in lateral view, straight
with expanded apexes of oblique upwards edges. Setae ventrally on the cerci (lateral view)
present in full extension. Apical shape of the cerci (last ⅓ portion of the cercus; in posterior
view) with the same size as the middle part. Conformation of the cerci in posterior view is
divergent. In lateral view, distiphallus with smooth margins ventroapically; distiphallic apical

120
conic shape, straight dorsal shape and presence of a small dorsoapical swelling. Pregonite with
expanded base and sudden narrowing at apex, which is darker than the base. Postgonite with
expanded base narrowing smoothly until the apex. Vesica asymmetric; terminal lobes reduced,
with filamentous at most tapering to apex shape and sclerotized texture; presence of spines
only along the edges; and presence of an angular median projection of the main vesica branch.
Remarks. See “remarks” section on O. afficta. Female was classified by Tibana &
Mello (1985), according to the shape of the T6+7, as syntergite partially divided into two
plates.
Distribution. NEOTROPICAL. Bolivia, Brazil (Ceará, Maranhão, Mato Grosso, Mato
Grosso do Sul, Pará, Rio de Janeiro), Colombia (Antioquia), Costa Rica, El Salvador,
Guatemala, Honduras, Panama, Peru, Venezuela.
Biology. This species has been reared on human feces and under laboratory conditions
(Lopes 1973b). Attractiveness by human feces, cow liver and lung, chicken viscera, pig
carcass, fish, crab, squid, rotten banana plus brown sugar, rotten Syagrus comosa (Mart.) Mart.
(Arecaceae) (a species of a palmtree – at coastal area) is reported for this species (Lopes
1973b; Lopes 1975a; Pamplona et al. 2000; Garcés et al. 2004; Barbosa et al. 2009; Ramírez-
Mora et al. 2012; Yepes-Gaurisas et al. 2013). As consequence of its higher frequency in
urban area, a high synanthropic index was obtained for O. timida in Antioquia, Colombia
(Yepes-Gaurisas et al. 2013).
Material examined. ♂: VENEZUELA: Aragua, Mouth of Rio Ocumare, costal flats
nr. La Boca, 17–20.xi.1997. T. Pape (ZMUC).
Oxysarcodexia trivialis (Wulp, 1895)
Sarcophaga trivialis Wulp, 1895
(Appendix 30-B)
Type locality. Mexico, Guerrero, Tierra Colorada, Amula, Xucumanatlan; Mexico,
Morelos, Cuernavaca; Mexico, Veracruz, Atoyac; Mexico, Tabasco, Teapa. Wulp, 1895: 268.
Depository of type material. BMNH.
Diagnosis. Male. Postocular plate with golden microtomentum. Ocellar setae not well-
developed. Thorax and abdomen with golden microtomentum more evident laterally.
Dorsocentral post-sutural setae with 2 well differentiated and 1–3 anterior setae smaller.

121
Apical scutellar seta present. Legs blackish. T3 with 3 lateral marginal seta. T4 with 1 median
marginal and 3 lateral marginal setae. T5 with golden microtomentum in part. ST5 with
parallel cleft edges and with setosity and setae on the apex of the arms. Cerci, in lateral view,
sinuous with expanded apexes of oblique upwards edges. Setae ventrally on the cerci (lateral
view) present at apical ⅓ portion. Apical shape of the cerci (last ⅓ portion of the cercus; in
posterior view) with the same size as the middle part. Conformation of the cerci in posterior
view is parallel. In lateral view, distiphallus with presence of a ventroapical concavity of
smooth margins; distiphallic apical rounded shape and straight distiphallic dorsal shape.
Pregonite with expanded base and sudden narrowing at apex, which is darker than the base.
Postgonite with expanded base narrowing smoothly until the apex. Vesica with symmetrical
branches; terminal lobes well-developed, with filamentous at most tapering to apex shape and
partially membranous texture; presence of spines only on ventral surface; and presence of a
rounded median projection of the main vesica branch.
Remarks. Vesica slender, without ornamentation and partially membranous is the
main characteristic which enables identification of O. trivialis with no doubt. See also
“remarks” section on O. edwardsi for further discussion. Female was classified by Tibana &
Mello (1985), according to the shape of the T6+7, as syntergite undivided.
Distribution. NEARTIC. Mexico (Districto Federal, Michoacán, Morelos, Puebla),
USA (Arizona, New Mexico). NEOTROPICAL. Costa Rica (Alajuela, Puntaneras, San José),
El Salvador, Guatemala, Mexico (Chiapas, Guerrero, Michoacán, Nuevo León, Sinaloa,
Tabasco, Veracruz), Panama.
Biology. Oxysarcodexia trivialis has been found breeding on human feces (Howard
1900). This species can also be an occasional flower visitor of avocado Persea americana Mill
(Castañeda-Vildózola et al. 1999).
Material examined. ♂♂: COSTA RICA: Puntaneras, Las Alturas, Cerro Chai, 2100m,
14.viii.1995, Th. Pape leg. (ZMUC) // COSTA RICA: San José, Rio Savegre, 9km SW route
2, San Gerardo de Dota 2200–2500m, 7–11.viii.1995, Th. Pape leg. (ZMUC).
Oxysarcodexia varia (Walker, 1836)
Sarcophaga varia Walker, 1836
Sarcophaga milleri Johnston & Tiegs, 1922

122
Sarcophaga chilensis Macquart, 1843
Sarcophaga flavicostata Macquart, 1843
Sarcophaga truncata Schiner, 1868
Sarcophaga nobilis Thomson, 1869
(Appendix 30-C)
Type locality. Uruguay, Gorrite Island, off Maldonado. Walker, 1836: 353.
Depository of type material. BMNH.
Diagnosis. Male. Postocular plate with golden microtomentum. Ocellar setae well-
developed. Thorax and abdomen with pale golden microtomentum more evident laterally.
Dorsocentral post-sutural setae with 3 well differentiated although a small bristle among these
3 can be present. Apical scutellar seta absent. Legs blackish. T3 with 1 lateral marginal seta.
T4 without median marginal and with 2 lateral marginal setae. T5 with golden microtomentum
in part. ST5 with V-shaped cleft edges and with setosity and setae along the edges of the arms.
Cerci, in lateral view, sinuous with expanded apexes of concave edges. Setae ventrally on the
cerci (lateral view) present in full extension. Apical shape of the cerci (last ⅓ portion of the
cercus; in posterior view) smaller than the middle part. Conformation of the cerci in posterior
view is divergent. Presence of a remarkable constriction at middle portion of the cerci, in
posterior view. In lateral view, distiphallus with smooth margins ventroapically; distiphallic
apical conic shape; sinuous distiphallic dorsal shape; and presence of a large dorsoapical
swelling on distiphallus. Pregonite with expanded base and sudden narrowing at apex and
same color along the entire extension. Postgonite with expanded base narrowing smoothly
until the apex. Vesica with asymmetrical branches; terminal lobes well-developed, with
rounded shape and sclerotized texture; presence of spines only along the edges; and presence
of a rounded median projection of the main vesica branch.
Remarks. See “remarks” section on O. augusta, O. aurata and O. marina.
Oxysarcodexia varia male and female were redescribed by Lopes & Albuquerque (1955);
characteristics as T7 interrupted medially, T8 compound by two plates without setosity,
ST7+8 small, with marginal setae and connected to ST8 which is well-developed, with golden
microtomentum and, on posterior margin, a deep longitudinal furrow were ascribed for
females.

123
Distribution. NEOTROPICAL. Argentina (Buenos Aires, Mendoza, Neuquén,
Tucumán), Bolivia, Brazil (Minas Gerais, Paraná, Rio de Janeiro, Rio Grande do Sul), Chile
(Bío Bío, Concepción, Coquimbo, Juan Fernandez Islands La Araucanía, Nuble, Patagonia,
Santiago, Valdivia), Uruguay (Corrite Island, Maldonado). AUSTRALASIAN/OCEANIAN.
Australia (New South Wales, Tasmania, Victoria), Fiji, French Polynesia (Society Islands),
New Zealand (North Island, South Island), Norfolk Island.
Biology. This species has been reared on dung (Mendes & Linhares 2002), dead
insects (Blanchard 1939) and in alive and dead lobsters (Blanchard 1939; Blanchard 1942;
Mulieri et al. 2010). This fly is attracted preferentially by feces, especially of dog (Mariluis et
al. 2007; Mulieri et al. 2010; Mulieri et al. 2011), but also by rotten minced kangaroo meat,
sheep‟s liver, carcasses (Meiklejohn et al. 2012) and rotten bovine liver (Mulieri et al. 2011).
Oxysarcodexia varia was more abundant during the spring and summer in Argentina and
Brazilian Southern and also considered asynanthropic due to higher abundance in rural areas
and grassland instead of woodland (Ferreira 1979; Mariluis et al. 2007; Mulieri et al. 2008;
Mulieri et al. 2010). Other ecological relationships of this species are the association as flower
visitor with Eryngium horridum (Spreng) Less (Apiaceae), Baccharis juncea (Cass) Desf,
Helianthus annuus L. (Asteraceae), Sebastiania brasiliensis Spreng (Euphorbiaceae), and
Colletia paradoxa (Spreng) Escal., Discaria americana Gillies & Hook, Scutia buxifolia
Reissek (Rhamnaceae) (Mulieri et al. 2010); and as host of Aleochara notula Erichson, 1839,
A. verberans Erichson, 1839 and A. puberula Klug, 1832 (Coleoptera: Staphilinidae) (Walsh
& Posse 2003). It has been collected in urban, suburban and rural areas (Mulieri et al. 2011).
This species has also a medical importance once it‟s considered a possible mechanical vector
of rabbit hemorrhagic disease virus (Henning et al. 2005).
Material examined. ♂: ARGENTINA, Chubut 47: Tecka, Corcovado, 750m
17.ii.1979, Mision Cientifica Danesa / NRM-DIPT 0014413 (NRM).
Oxysarcodexia ventricosa (Wulp, 1895)
Sarcophaga ventricosa Wulp, 1895
Sarcophaga tenuiventris Wulp, 1895
(Appendix 31-A)

124
Type locality. Mexico, Guerrero, Chilpancingo; Mexico, Tabasco, Teapa. Wulp, 1895:
268.
Depository of type material. BMNH.
Diagnosis. Male. Postocular plate with silveryish microtomentum. Ocellar setae not
well-developed. Thorax and abdomen with golden microtomentum more evident laterally.
Dorsocentral post-sutural setae with 2 well differentiated and 1–3 anterior setae smaller.
Apical scutellar seta absent. Legs yellowish. T3 with 3 lateral marginal setae. T4 with 1
median marginal and 2 lateral marginal setae. T5 with golden microtomentum partially. ST5
with parallel cleft edges and with setosity and setae on the apex of the arms. Cerci, in lateral
view, straight with expanded apexes of oblique upwards edges. Setae ventrally on the cerci
(lateral view) present on apical ⅓ portion. Apical shape of the cerci (last ⅓ portion of the
cercus; in posterior view) bigger than the middle part. Conformation of the cerci in posterior
view is divergent. Presence of a remarkable constriction at middle portion of the cerci, in
posterior view. In lateral view, distiphallus with presence of a ventroapical concavity of
serrated margins; distiphallic apical conic shape and straight distiphallic dorsal shape.
Pregonite with expanded base narrowing smoothly until the apex, which is darker than the
base; same as postgonite except for the color which is the same along the entire extension.
Vesica with symmetrical branches; terminal lobes reduced, with filamentous at most tapering
to apex shape and sclerotized texture; presence of spines on both dorsal and ventral surfaces;
and presence of a rounded median projection of the main vesica branch.
Remarks. See O. admixta “remarks” section. Ravinia assidua (Walker, 1853) and O.
ventricosa have already been involved in a taxonomical issue as pointed out by Dodge
(1956b). Female was classified by Tibana & Mello (1985), according to the shape of the T6+7,
as syntergite undivided. Larvae are known and were described by Knipling (1936) and
Wharton & Moon (1979). For further comments about the larvae, see “remarks” section on O.
galeata. This species has been intercepted in imported container with cargoes from Americas
in Shanghai Port, China (Deng et al. 2011).
Distribution. NEARTIC. Bermuda, Canada (Ontario, Quebec), Mexico (Districto
Federal, Morelos, San Luis Potosí), USA (Alabama, Arkansas, District of Columbia, Florida,
Georgia, Illinois, Indiana, Iowa, Louisiana, Maryland, Massachusetts, Missouri, Nebraska,
New Jersey, New York, Ohio, Oklahoma, Pennsylvania, South Carolina, Texas, West

125
Virginia). NEOTROPICAL. Argentina (Córdoba), Costa Rica, Honduras, Mexico (Chiapas,
Guerrero, Tabasco, Veracruz, Yucatán). PALEARTIC. China (Shangai).
Biology. This species has been rearing mainly on bovine dung (Knipling 1936; Sanders
& Dobson 1966; Blume 1986). Larvae were reared on cow manure and, in one case, on
decomposing hamburger meat, took 14 days to complete the developmental period, from larva
to adult (Knipling 1936). When immatures were reared from a bovine pad, they took 15–25
days to complete the development until the emergence of adults (Sanders & Dobson 1966).
Larvae were also recorded feeding on eggs and larvae of Oxybelus wasp, probably as an
alternative choice due to scarcity of the preferential resource (Bohart et al. 1966; Peckham
1991), thus it‟s considered a species probably capable of prey-parasitism (Peckham 1991).
Oxysarcodexia ventricosa is considered a scavenger species, being collected associated to pig
carcass exposed on tree and water environments (Payne & King 1972) and in the active decay
stage of decomposition (Tabor et al. 2004; Tabor et al. 2005). This dung-breeding species has
been collected perching on pitcher plants (Dahlem & Naczi 2006), on high lands (Dodge &
Seago 1954) and in a bog (Judd 1970). Pupae originated from larvae bred on dung were
parasitized by Aphaereta pallipes (Say) (Hymenoptera: Braconidae) (Figg et al. 1983). This
fly was one of the species used to study the phylogeny of Sarcophagidae based on a molecular
approach (mtDNA fragments) (Stamper et al. 2012).
Material examined. ♂♂: ALABAMA: Madison Co. 10km NE Maysville, Sharp
Cove, Sneed Spg. bottomland, 240m, 6 oct 1992, Acciavath / NRM-DIPT 0014456 (NRM) //
USA, Fla, Highlands Co., Archbold Biol. St., 15.vi.1989, T. Pape leg. (ZMUC).
Oxysarcodexia villosa Lopes, 1946
(Appendix 31-B)
Type locality. Brazil, Pará. Lopes, 1946a: 458.
Depository of type material. BMNH.
Diagnosis. Male. Postocular plate with golden microtomentum. Ocellar setae not well-
developed. Thorax with pale golden microtomentum. Dorsocentral post-sutural setae with 2
well differentiated and 2 anterior setae smaller. Apical scutellar seta absent. Legs blackish.
Abdomen with silvery microtomentum, although some shades of pale golden microtomentum
is seen laterally. T3 with 1 lateral marginal seta. T4 with 1 median marginal and 2 lateral

126
marginal setae. T5 with golden microtomentum in part. ST5 with parallel cleft edges and with
setae scattered on arm‟s surface. Cerci, in lateral view, straight with normal apexes (same size
as the median area) of oblique upwards edges. Setae ventrally on the cerci (lateral view)
present in apical ⅓ portion. Apical shape of the cerci (last ⅓ portion of the cercus; in posterior
view) with the same size as the middle part. Conformation of the cerci in posterior view is
parallel. Presence of a remarkable constriction at middle portion of the cerci, in posterior view.
In lateral view, distiphallus with smooth margins ventroapically; distiphallic apical shape
rounded and straight distiphallic dorsal shape. Pregonite and postgonite with expanded base
narrowing smoothly until the apex and same color along the entire extension. Vesica with
asymmetrical branches; terminal lobes well-developed, with oblong shape and sclerotized
texture; presence of spines on basal half of ventral surface and on the edge of the right lobe
(more developed than the left lobe); and presence of an angular median projection of the main
vesica branch.
Remarks. This is another species of this genus that have a noteworthy asymmetrical
vesica. In O. villosa, vesica is very developed, surpassing substantially the distiphallus apex.
Distribution. NEOTROPICAL. Brazil (Pará).
Biology. Unknown.
Material examined. ♂: Inst. Agronômico do Norte, Belém, Est. Do Pará – Brasil XI-
959. L. Trav. D. Lacombe J. Evangelista E. Lobato / Oxysarc villosa ♂ Lop det. H. S. Lopes
(MNRJ).
Oxysarcodexia vittata (Walker, 1836)
Sarcophaga vittata Walker, 1836
Oxysarcodexia titubata Lopes, 1946
(Appendix 31-C)
Type locality. South America. Walker, 1836: 353.
Depository of type material. BMNH.
Diagnosis. Male. Postocular plate with golden microtomentum. Ocellar setae well-
developed. Thorax with golden microtomentum. Dorsocentral post-sutural setae with 3 well
differentiated, a small bristle among these 3 can be present. Apical scutellar seta present. Legs
blackish. Abdomen with silvery microtomentum. T4 with 2 lateral marginal setae. T5 with

127
golden microtomentum in part. ST5 with parallel cleft edges and with setosity and setae on the
apex of the arms. Cerci, in lateral view, sinuous with pointed apexes of oblique upwards
edges. Setae ventrally on the cerci (lateral view) present in full extension. Apical shape of the
cerci (last ⅓ portion of the cercus; in posterior view) smaller than the middle part.
Conformation of the cerci in posterior view is divergent. In lateral view, distiphallus with
smooth margins ventroapically; distiphallic apical shape square/oblong; sinuous distiphallic
dorsal shape; presence of ventroapical projections, lateral lobes, and a medium dorsoapical
swelling on distiphallus. Presence of lateroapical expansions on distiphallus, in dorsal view.
Pregonite with base and apex with same size and apex darker than the base. Postgonite with
expanded base and sudden narrowing at apex and same color along the entire extension.
Vesica symmetric; terminal lobes well-developed, with square shape and sclerotized texture;
and without spines.
Remarks. See “remarks” section on Oxysarcodexia n. sp. 4, O. augusta and O. nitida.
Female is characterized by T7 entire, sclerotized dorsally and with the presence of a large
membrane connecting it to T5; T8 constituted by two elongated plates without setae; ST6
larger than ST5 and with apical setae; ST7 larger than long and connected to ST8 by an
extensive membranous region (Lopes 1946a).
Distribution. NEOTROPICAL. Brazil (Mato Grosso, Mato Grosso do Sul, Rio de
Janeiro, Paraná, Santa Catarina, São Paulo).
Biology. This species is attracted by human feces and had already been reared on
laboratory (Lopes 1973b), although no further information was reported.
Material examined. ♂♂: BRAZIL: São Paulo, Jundiaí, Serra do Japi; 06.I.2012; M.
D. Grella / Oxysarcodexia 01; Jundiaí-SP; 06/01/2012 / O. titubata (L2B-DBA) // Brasilien,
Nova Teutonia, 27°11‟B 52°23‟L, Fritz Plaumann / Xarcophaga titubata (Lopes) /
Oxysarcodexia (ZMUC).
Oxysarcodexia wygodzinskyi Lopes & Tibana, 1987
(Appendix 32-A)
Type locality. Argentina, Salta, San Martin. Lopes & Tibana, 1987: 336.
Depository of type material. MNRJ.

128
Diagnosis. Male. Postocular plate with silvery microtomentum. Ocellar setae well-
developed. Thorax with pale golden microtomentum on humeral region. Dorsocentral post-
sutural setae with 2 well differentiated and 3 anterior setae smaller. Apical scutellar seta
absent. Legs blackish. Abdomen with silvery microtomentum. T3 with 2 lateral marginal
setae. T4 with 1 median marginal and 2 lateral marginal setae. T5 with silvery
microtomentum, although some pale golden microtomentum on anterior margin is seen. ST5
with parallel cleft edges and with setae on the apex of the arms. Cerci, in lateral view, sinuous
with pointed apexes of oblique upwards edges. Setae ventrally on the cerci (lateral view)
present in apical half portion. Apical shape of the cerci (last ⅓ portion of the cercus; in
posterior view) smaller than the middle part. Conformation of the cerci in posterior view is
divergent. In lateral view, distiphallus with ventroapical concavity with smooth margins
ventroapically; distiphallic apical shape rounded and straight distiphallic dorsal shape.
Pregonite with base and apex with same size and sudden narrowing at apex and same color
along the entire extension. Postgonite with expanded base narrowing smoothly until the apex
and same color along the entire extension. Vesica symmetric; terminal lobes well-developed,
with rounded shape and sclerotized texture; presence of spines only apically along the edges.
Remarks. See “remarks” section on O. cingarus.
Distribution. NEOTROPICAL. Argentina (Salta), Brazil (Mato Grosso, Mato Grosso
do Sul).
Biology. Unknown.
Material examined. ♂: Oxysarcodexia wygodzinsky Lopes & Tibana, 1987, det. C. A.
Mello-Patiu / BRASIL, MS, Porto Murtinho, Fazenda Retiro Conceição S21°41‟18.8”
W057°45‟53.7” Coleta manual (rede) 11.xii.2011 Patiu & Patiu col. (MNRJ).
Oxysarcodexia xanthosoma (Aldrich, 1916)
Sarcophaga xanthosoma Aldrich, 1916
(Appendix 32-B)
Type locality. Guatemala, Los Amates. Aldrich, 1916: 274.
Depository of type material. USNM.
Diagnosis. Male. Postocular plate with pale golden microtomentum. Ocellar setae not
well-developed. Thorax and abdomen with intense golden microtomentum. Dorsocentral post-

129
sutural setae with 2 well differentiated and 1–3 anterior setae smaller. Apical scutellar seta
present. Legs blackish. T3 with 3 lateral marginal setae. T4 with 1 median marginal and 2
lateral marginal setae. T5 with golden microtomentum in part. ST5 with parallel cleft edges
and setosity. Cerci, in lateral view, straight with expanded apexes of oblique upwards edges.
Setae ventrally on the cerci (lateral view) absent only in the middle portion. Apical shape of
the cerci (last ⅓ portion of the cercus; in posterior view) smaller than the middle part.
Conformation of the cerci in posterior view is divergent. In lateral view, distiphallus with
presence of a ventroapical concavity of smooth margins; distiphallic apical rounded shape;
sinuous distiphallic dorsal shape. Pregonite with expanded base and sudden narrowing at apex,
which is darker than the base. Postgonite with expanded base narrowing smoothly until the
apex and same color along the entire extension. Vesica with symmetrical branches; presence
of lateral lobes (i.e. division of the vesica coming from or close to the basal branch of the
vesica, placed laterally to phallic tube); terminal lobes well-developed, with filamentous at
most tapering to apex shape and sclerotized texture; presence of spines only on ventral
surface; and presence of a rounded median projection of the main vesica branch.
Remarks. This species can exhibit high morphological variation, especially in cerci
and phallus apical portions and also on microtomentum, as pointed out by Lopes (1975c),
leading to misidentification of O. xanthosoma as O. amorosa. See “remarks” section on
Oxysarcodexia n. sp. 1. Female was classified by Tibana & Mello (1985), according to the
shape of the T6+7, as syntergite undivided.
Distribution. NEARTIC. Mexico (San Luis Potosí, Sonora). NEOTROPICAL.
Argentina (Misiones), Brazil (Amapá, Amazonas, Ceará, Espírito Santo, Mato Grosso, Minas
Gerais, Pará, Paraná, Rio de Janeiro, Roraima, São Paulo), Colombia, Costa Rica, Ecuador, El
Salvador, Guatemala (Los Amates), Guyana (Kartabo), Mexico (Jalisco, Veracruz), Panama,
Peru.
Biology. This species has already been reared under laboratory conditions (Lopes
1973b) and on human feces (D‟Almeida 1994). This fly has been collected using human feces,
peccary entrails, rotting liver, fish, pig carcass, banana plus brown sugar, rotten Syagrus
comosa (Mart.) Mart. (Arecaceae), a species of a palmtree (at coastal area) as baits (Curran &
Walley 1934; Lopes 1973b; Lopes 1975a; Oliveira et al. 2002; Leandro & D‟Almeida 2005;
Barbosa et al. 2009; Rosa et al. 2011; Vairo et al. 2011). The presence of O. xanthosoma is

130
known for urban (zoological garden), Cerrado (“savanna-like”) vegetation, peri-urban and
forest areas (Lopes & Tibana 1982; Oliveira et al. 2002; Rosa et al. 2011; Vairo et al. 2011).
Material examined. ♂♂: ECUADOR: Napo Province: Yasuní National Park: Yasuní
Research Station: 76°36‟W 00°38‟S 3–20.XI.1998 T. Pape & B. Viklund / NRM-DIPT
0014476 / O. xanthosoma (NRM) // Oxysarcodexia xanthosoma / Campinas, SP VII/2012
Brancoli DL (L2B-DBA) // Oxysarcodexia xanthosoma / Campinas, SP VII/2012 Brancoli DL
(L2B-DBA) // Oxysarcodexia xanthosoma / Campinas, SP VII/2012 Brancoli DL (L2B-DBA).
Oxysarcodexia xon (Dodge, 1968)
Xarcophaga xon Dodge, 1968
(Appendix 32-C)
Type locality. Panama, Canal Zone, Barro Colorado Island. Dodge, 1968: 449.
Depository of type material. KU.
Diagnosis. Male. Postocular plate pale golden microtomentum. Ocellar setae not well-
developed. Thorax and abdomen with silveryish microtomentum. Three well differentiated
dorsocentral post-sutural setae. Apical scutellar seta is absent. T3 with median marginal setae.
T5 with yellowish microtomentum more evident laterally. T4 with median marginal setae. ST5
with V-shaped cleft edges and short apical prolongations. Cerci with straight shape (lateral
view) with a small anterior point. Phallus entire with the presence of an expanded apical
serrated plate and lateral serrated plates. Small vesica. This is a synopsis of the original
description given by Dodge (1968) and of the diagnosis given by Lopes (1975c).
Remarks. See “remarks” section on Oxysarcodexia n. sp. 4 and O. notata.
Distribution. NEOTROPICAL. Brazil (Rio de Janeiro), Panama (Barro Colorado
Island, Canal Zone).
Biology. Unknown.
Oxysarcodexia zayasi Dodge, 1956
(Appendix 32-D)
Type locality. Cuba, Habana, Lomas de Camoa. Dodge, 1956: 100.
Depository of type material. USNM.

131
Diagnosis. Male. Postocular plate with pale golden microtomentum. Ocellar setae not
well-developed. Thorax with slightly pale golden microtomentum. Dorsocentral post-sutural
setae with 2 well differentiated and 1–3 anterior setae smaller. Apical scutellar seta absent.
Legs yellowish. Abdomen with slightly silveryish microtomentum. T3 with 3 lateral marginal
setae. T4 without median marginal and with 2 lateral marginal setae. T5 without golden
microtomentum, but silveryish microtomentum shades are present. ST5 with V-shaped cleft
edges and with setosity. Cerci, in lateral view, straight with expanded apexes of oblique
upwards edges. Setae ventrally on the cerci (lateral view) present in full extension. Apical
shape of the cerci (last ⅓ portion of the cercus; in posterior view) bigger than the middle part.
Conformation of the cerci in posterior view is parallel. Presence of a remarkable constriction
at middle portion of the cerci, in posterior view. In lateral view, distiphallus with presence of a
ventroapical concavity of smooth margins; distiphallic apical square/oblong shape; and
straight distiphallic dorsal shape. Pregonite with expanded base narrowing smoothly until the
apex, which is darker than the base. Postgonite with expanded base and sudden narrowing at
apex and same color along the entire extension. Vesica with symmetrical branches; terminal
lobes reduced, with filamentous at most tapering to apex shape and partially membranous
texture; presence of spines only on ventral surface; and presence of a rounded median
projection of the main vesica branch.
Remarks. See “remarks” section on O. cyanea. Female was classified by Tibana &
Mello (1985), according to the shape of the T6+7, as syntergite undivided.
Distribution. NEOTROPICAL. Bahamas (Andros, Eleuthera, Grand Bahama, New
Providence), Cuba (Havana, Lomas de Camoa).
Biology. Unknown. Type specimens were collected with fish head exposed in a
wooded area (Dodge 1956).
Material examined. ♂♂: KUBA: Provinz Santiago de Kuba; Cordillera de La Gran
Piedra [unreadable information] 75°38‟W, 20°02‟N, [unreadable information] 19–26.XII.1997
Leg. Stark / Oxysarcodexia zayasi Dodge, det. T. Pape 1998-08-27 / NRM-DIPT 0014463
(NRM) // Cuba prov. Las Villas Trinidad 100m 29.VI.196[0 or 6 – damaged by the pinhole]
leg. F. Gregor / Oxysarcodexia zayasi Dodge B. Rohdendorf det 1970. I (MNRJ).

132
Final considerations
Similarities on male terminalia of very close related species, the notable number of
species presenting asymmetry on the vesica, in comparison to other genus of Sarcophagidae,
and a better comprehension of “Xarcophaga”, “paulistanensis”, “ventricosa” and “thornax”
groups call for a thorough investigation, in order to provide a phylogenetic approach for
clarify these issues. Furthermore, it is also needed a deeper study of females and larval
morphology, besides ultrastructural analysis of male terminalia (e.g. scanning electron
microscopy) and molecular approaches for a wider and more complete understanding of
relationships inside the genus Oxysarcodexia.
Thus, this taxonomic conspectus is an initial wider step towards Oxysarcodexia
comprehension, contributing with an update of the male knowledge of species within this
genus, their morphological peculiarities and geographical distribution, also gathering
information about their biology, which is still incipient. Also, pictorial information here
documented, together the presented diagnosis, enable a comparison and, consequently, an
accurate identification until the level of species, particularly important in the case of close
related species and living in sympatry.
Acknowledgments
To Dr. Cátia Antunes de Mello-Patiu for provided the means for the examination of
specimens from the Museu Nacional/Universidade Federal do Rio de Janeiro; MsC. Eliana
Buenaventura for provided the means for the examination of specimens from the Tecnológico
de Antioquia, Institución Universitaria; and Dr. Kjell Arne Johanson for provided the means
for the examination of specimens from the Swedish Museum of Natural History for loan of
material.
This research was supported by CAPES Foundation, Ministry of Education of Brazil
(grant #0906/12-3).
References
Aldrich, J.M. (1916) Sarcophaga and allies in North America. Thomas Say Foundation, Entomological
Society of America, La Fayette, Indiana, 301 pp. + 16 pls.

133
Aldrich, J.M. (1930) Notes on the types of American two-winged flies of the genus Sarcophaga and a
few related forms, described by the early authors. Proceedings of the United States National
Museum, 78, 1–39 + 3 pls. http://dx.doi.org/10.5479/si.00963801.78-2855.1
Amat, E. (2010) Notes on necrophagous flies (Diptera: Calyptratae) associated to fish carrion in
Colombian Amazon. Acta Amazonica, 40 (2), 397–400.
Barbosa, R.R., Mello-Patiu, C.A., Mello, R.P. & Queiroz, M.M.C. (2009) New records of calyptrate
dipterans (Faniidae, Muscidae and Sarcophagidae) associated with the decomposition of domestic
pigs in Brazil. Memórias do Instituto Oswaldo Cruz, 104 (6), 923–926.
Barros, R.M. de, Mello-Patiu, C.A. de & Pujol-Luz, J.R. (2008) Sarcophagidae (Insecta, Diptera)
associados à decomposição de carcaças de Sus scrofa Linnaeus (Suidae) em área de Cerrado do
Distrito Federal, Brasil. Revista Brasileira de Entomologia, 52 (4), 606–609. [In Portuguese]
http://dx.doi.org/10.1590/S0085-56262008000400011
Beuter, L., Fernandes, P.A., Barros, P.B., Souza, C.R. & Mendes, J. (2012) Insetos de potencial
importância forense e na saúde pública em região urbana de Minas Gerais: frequência relativa e
variação sazonal de fauna atraída e criada em carcaças de roedores. Revista de Patologia Tropical,
41 (4), 480–490. [In Portuguese]
Blanchar, E.E. (1935) Contribución al estúdio de los sarcofágidos argentinos. Physis, 11, 508. [In
Spanish]
Blanchard, E.E. (1939) Los sarcofágidos argentinos. Contribución a su conocimiento. Physis, 17, 791–
856. [In Spanish]
Blanchard, E.E. (1942) Parasitos de Alabama argillacea Hbn. en la República Argentina. Estudio
preliminar. Anales de la Sociedad Científica Argentina, 134, 54–63. [In Spanish]
Blume, R.R. (1985) A checklist, distribution record, and annotated bibliography of the insects
associated with bovine droppings on pastures in America north of Mexico. The Southwestern
Entomologist, 9, 1–28.
Blume, R.R. (1986) Parasites of Diptera associated with bovine droppings on a pasture in east central
Texas. The Southwestern Entomologist, 11 (3), 215–222.
Boff, S., Graciolli, G., Boaretto, A.G. & Marques, M.R. (2008) Insetos visitantes de gomas exsudadas
por Terminalia argentea Marct & Zucc (Combretaceae). Revista Brasileira de Entomologia, 52
(3), 477–479. [In Portuguese]
Bohart, R.M., Lin, C.S. & Holland, J.F. (1966) Bionomics of Oxybelus sparideus at Lake Texoma,
Oklahoma (Hymenoptera: Sphecidae: Craboninae). Annals of the Entomological Society of
America, 59 (4), 818–820.

134
Brown, B.V. (2009) Introduction. In: Brown, B.V., Borkent, A., Cumming, J.M., Wood, D.M.,
Woodley, N.E. & Zumbado, M.A. (eds.), Manual of American Central Diptera. Vol. 1. NCR
Research Press, Ottawa, Ontario, Canada, pp. 1–7.
Carvalho, C.J.B. & Mello-Patiu, C.A. (2008) Key to the adults of the most common forensic species of
Diptera in South America. Revista Brasileira de Entomologia, 52, 390–406.
http://dx.doi.org/10.1590/S0085-56262008000300012
Carvalho, C.J.B., Rafael, J.A., Couri, M.S. & Silva, V.C. (2012) Diptera. In: Rafael, J.A., Melo,
G.A.R., Carvalho, C.J.B., Casari, S. & Constantino, C. (eds.), Insetos do Brasil: diversidade e
taxonomia. Holos Editora, Ribeirão Preto, São Paulo, Brasil, pp. 701–743. [In Portuguese]
Carvalho, L.M.L. & Linhares, A.X. (2001) Seasonality of insect succession and pig carcass
decomposition in a natural forest area in southeastern Brazil. Journal of Forensic Sciences, 46 (3),
604–608.
Castañeda-Vildózola, A., Equihua-Martínez, A., Valdés-Carrasco, J., Barrientos-Priego, A.F., Ish-Am,
G. & Gazit, S. (1999) Insectos polinizadores del aguacareto em los estados de México y
Michoacán. Revista Chapingo Serie Horticultura, 5, 129–136. [In Spanish]
Costamagna, R., Visciarelli, E.C, Lucchi, L.D., Basabe, N.E., Esteban, M.P. & Oliva, A. (2007)
Aportes al conocimiento de los dípteros ciclorrafos em el área urbana de Bahía Blanca (província
de Buenos Aires), Argentina. Revista del Museo Argentino de Ciencias Naturales, 9 (1), 1–4.
Cornaby, B.W. (1974) Carrion reduction by animals in contrasting tropical habitats. Biotropica, 6 (1),
51–63.
Couri, M.S., Barros, G.P.S., Orsini, M.P. (2008) Dipterofauna do Arquipélago de Fernando de
Noronha (Pernambuco, Brasil). Revista Brasileira de Entomologia, 52 (4), 588–590. [In
Portuguese]
Couri, M.S., Lamas, C.J.E., Aires, C.C.C., Mello-Patiu, C.A., Pamplona, D.M. & Magno, P.D. (2000)
Diptera from Serra do Navio (Amapá, Brasil): Asilidae, Bombyliidae, Calliphoridae,
Micropezidae, Muscidae, Sarcophagidae, Stratiomyiidae, Syrphidae, Tabanidae and Tachinidae.
Revista Brasileira de Zoociências, 2 (1), 91–100.
Cruz, T. M. (2008) Diversidade e sucessão ecológica de insetos associados à decomposição animal em
um fragmento de Mata Atlântica de Pernambuco. Dissertation. Recife, Pernambuco, Brasil.
102pp. [In Portuguese]
Curran, C.H. & Walley, G.S. (1934) Sarcophagidae. In: Curran, C.H. The Diptera of Kartabo, Bartica
District, British Guiana, with descriptions of new species from other British Guiana localities.
Bulletin of the American Museum of Natural History, 66, pp. 287–532.

135
D‟Almeida, J.M. & Lima, S.F. (1994) Atratividade de diferentes iscas e sua relação com as fases de
desenvolvimento ovariano em Calliphoridae e Sarcophagidae (Insecta, Diptera). Revista
Brasileira de Zoologia, 11(2), 177–186. [In Portuguese]
D‟Almeida, J.M. (1984) Sinantropia de Sarcophagidae (Diptera) na região metropolitana do Estado do
Rio de Janeiro. Arquivos da Universidade Federal Rural do Rio Janeiro, 7, 101-110. [In
Portuguese]
D‟Almeida, J.M. (1986) Substratos para criação de dípteros caliptratos em área rural do Estado do Rio
de Janeiro. Arquivos da Universidade Federal Rural do Rio de Janeiro, 9 (1–2): 13–22. [In
Portuguese]
D‟Almeida, J.M. (1988) Substratos utilizados para a criação de dípteros caliptratos em uma área urbana
do município do Rio de Janeiro. Memórias do Instituto Oswaldo Cruz, 83, 201–206. [In
Portuguese] http://dx.doi.org/10.1590/S0074-02761988000200009
D‟Almeida, J.M. (1989) Substratos utilizados para a criação de dípteros caliptratos no jardim zoológico
do Rio de Janeiro. Memórias do Instituto Oswaldo Cruz, 84, 257–264. [In Portuguese]
http://dx.doi.org/10.1590/S0074-02761989000200016
D‟Almeida, J.M. (1994) Ovipositional substrates used by Calyptrate Diptera in Tijuca Forest, Rio de
Janeiro. Memórias do Instituto Oswaldo Cruz, 89 (2), 261–264.
Dahlem, G.A. & Naczi, R.F.C. (2006) Flesh flies (Diptera: Sarcophagidae) associated with North
American pitcher plants (Sarraceniaceae), with descriptions of three new species. Annals of the
Entomological Society of America, 99 (2), 218–240.
Deng, Y.H., Wang, G.P., Chen Z.Z & Fan Z.D. (2011) Three species of the genus Oxysarcodexia
Townsend, 1917 (Diptera: Sarcophagidae) intercepted in imported container with cargoes from
Americas in Shanghai Port. Acta Parasitologica et Medica Entomologica Sinica, 18 (1), 42–47.
doi:10.3969/j.issn.1005-0507.201.1.01.009 [In Chinese]
Dias, E.S., Neves, D.P. & Lopes, H.S. (1984a) Estudos sobre a fauna de Sarcophagidae (Diptera) de
Belo Horizonte – Minas Gerais I – Levantamento taxonômico e sinantrópico. Memórias do
Instituto Oswaldo Cruz, 79 (1), 83–91. [In Portuguese]
Dias, E.S., Neves, D.P. & Lopes, H.S. (1984b) Estudos sobre a fauna de Sarcophagidae (Diptera) de
Belo Horizonte – Minas Gerais II – Variação Sazonal. Memórias do Instituto Oswaldo Cruz, 79
(4), 409–412. [In Portuguese]
Dias, E.S., Neves, D.P. & Lopes, H.S. (1984c) Estudos sobre a fauna de Sarcophagidae (Diptera) de
Belo Horizonte – Minas Gerais II – Atratividade das Iscas. Memórias do Instituto Oswaldo Cruz,
79 (4), 413–417. [In Portuguese]
Dodge, H.R. & Seago, J.M. (1954) Sarcophagidae and other Diptera taken by trap and net on Georgia
Mountain Summits in 1952. Ecology, 35 (1), 50–59.

136
Dodge, H.R. (1956) Two new Sarcophagid flies from Cuba (Diptera). Memórias de la Sociedad
Cubana de Historia Natural, 23 (1), 97–103.
Dodge, H.R. (1965) The Sarcophagidae (Diptera) of the West Indies. II. Jamaica. Annals of the
Entomological Society of America, 58, 497–517.
Dodge, H.R. (1966) Some new or little-known Neotropical Sarcophagidae (Diptera), with a review of
the genus Oxysarcodexia. Annals of the Entomological Society of America, 59, 674–701.
Dodge, H.R. (1968) The Sarcophagidae of Barro Colorado Island, Panama (Diptera). Annals of the
Entomological Society of America, 61 (2), 421–450.
Enderlein, G. (1928) Klassifikation der Sarcophagiden. Sarcophagiden-Studien I. Archiv für
Klassifikatorische und Phylogenetische Entomologie, Wien, 1, 1–56. [In German]
Engel, O. (1931) Die Ausbeute der deutschen Chaco –Expedition 1925/1926 – Diptera XXVI.
Anthomyidae, XXVII. Muscidae und XXVIII. Sarcophagidae. Konowia, 10 (2), 133–154. [In
German]
Evenhuis, N.L., Pape, T., Pont, A.C. & Thompson, F.C. (eds.) (2007) Biosystematic Database of
World Diptera, Version 10. Available at http://www.diptera.org/biosys.htm. apud Pape, T.,
Bickel, D. & Meier, R. (2009) Diptera Diversity: status, challenges and tools. Brill, Portland,
459pp.
Ferreira, M.J.M. (1979) Sinantropia de Dipteros Muscoides de Curitiba. II: Sarcophagidae. Revista
Brasileira de Biologia, 39 (4), 773–781. [In Portuguese]
Ferreira, M.J.M., Rizzo, J.A., Rasmussen, G., Pereira, I.F. (1980) Ocorrência e frequência de
califorídeos em formações de mata e cerrado no município de Goiânia-Goiás. Anais da E.A.V., 1,
13–26. [In Portuguese]
Figg, D.E., Hall, R.D. & Thomas, G.D. (1983) Host range and eclosion success of the parasite
Aphaereta pallipes (Hymenoptera: Braconidae) among dung-breeding Diptera in Central
Missouri. Enviromental Entomology, 12 (3), 993–995.
Figueiredo, R.R., Dorf, S., Couri, M.S., Azevedo, A.A. & Mossumez, F. (2002) Corpos estranhos
animados em otorrinolaringologia. Revista Brasileira de Otorrinolaringologia, 68 (5), 722–728.
Flores, V.I. & Dale, W.E. (1995) Um estúdio sobre ecologia de las moscas Sarcophagidae em la costa
central peruana. Revista Peruana de Entomología, 38, 13–17. [In Spanish]
Garcés, P.A., Bermudes, S. & Quintero, G. (2004) Determinación de la entomofauna associada a
carcaças de cerdos domésticos vestidos (Sus scrofa), en el Puerto de Vacamonte, Prov. de
Panamá. Tecnociencia, 6 (2), 59–74. [In Spanish]

137
García-Franco, J.G. & Rico-Gray, V. (1997) Reproductive biology of the holoparasitic endophyte
Bdallophyton bamburarum (Rafflesiaceae). Botanical Journal of the Linnean Society, 123, 237–
247.
Giroux, M., Pape, T. & Wheeler, T.A. (2010) Towards a phylogeny of the flesh flies (Diptera:
Sarcophagidae): morphology and phylogenetic implications of the acrophallus in the subfamily
Sarcophaginae. Zoological Journal of the Linnean Society, 158, 740–778.
http://dx.doi.org/10.1111/j.1096-3642.2009.00561.x
Hall, D.G. (1933) The Sarcophaginae of Panama (Diptera: Calliphoridae). Bulletin of the American
Museum of Natural History, 66, 251–285.
Hall, D.G. (1937) New muscoid flies (Diptera) in the United States National Museum. Proceedings of
the United States National Museum, 84, 201–216. http://dx.doi.org/10.5479/si.00963801.84-
3011.201
Hall, D.G. (1938) New genera and species of South American Sarcophagidae (Diptera). Arbeiten über
Morphologische und Taxonomische Entomologie aus Berlin-Dahlem, 5, 253–259.
Henning, J., Schnitzler, F.R., Pfeiffer, D.U. & Davies, P. (2005) Influence of weather conditions on fly
abundance and its implications for transmission of rabbit hemorrhagic disease virus in the North
Island of New Zealand. Medical and Veterinary Entomology, 19, 251–262.
Horenstein, M.B., Linhares, A.X., Ferradas, B.R. & García, D. (2010) Decomposition and dipteran
succession in pig carrion in central Argentina: ecological aspects and their importance in forensic
science. Medical and Veterinary Entomology, 24, 16–25.
Howard, L.O. (1900) A Contribution to the study of the insect fauna of human excrement. Proceedings
of the Washington Academy of Science, 2, 541–604.
Johnston, T.H. & Tiegs, C.W. (1922) Sarcophagidae flies in the Australian Museum collection.
Records of the Australian Museum. 13: 175–188.
Judd, W.W. (1970) Studies on the Byron Bog in Southwestern Ontario XLIV flies (Sarcophagidae,
Muscidae and Tachinidae) trapped in the Bog. Entomology News, 81, 189–190.
Knipling, E.F. (1936) A comparative study of the first-instar larvae of the genus Sarcophaga
(Calliphoridae, Diptera), with notes on the biology. The Journal of Parasitology, 22 (5), 417–454.
Kunz, S.E. (1978) Notes on the seasonal activity of dung infesting Diptera in Central Texas. The
Southwestern Entomologist, 3 (3), 167–170.
Leandro, M.J.F. & D‟Almeida, J.M. (2005) Levantamento de Calliphoridae, Fanniidae, Muscidae e
Sarcophagidae em um fragmento de mata na Ilha do Governador, Rio de Janeiro, Brasil.
Iheringia, Série Zoologia, 95 (4) 377–381. [In Portuguese]

138
Lehrer, A.Z. & Barbet, A. (2008) Sarcophagides de la faune de Nouvelle-Calédonie (Diptera,
Sarcophagidae). Fragmenta Dipterologica, 17: 1–30. [In French]
Leite, A.C.R. & Lopes, H.S. (1987) Second contribution to the knowledge of the larvae of the
Raviniini (Diptera, Sarcophagidae) based on observations using scanning electron microscope.
Memórias do Instituto Oswaldo Cruz, 82 (2), 219–226.
Linhares, A.X. (1981) Synanthropy of Calliphoridae and Sarcophagidae (Diptera) in the city of
Campinas, São Paulo, Brazil. Revista Brasileira de Entomologia, 25, 189–215.
Lopes, H.S. & Albuquerque, D.O. (1955) Los insectos de las Islas Juan Fernandez. Calliphoridae et
Sarcophagidae (Diptera). Revista Chilena de Entomología, 4, 3–20.
Lopes, H.S. & Leite, A.C.R. (1986) Studies on some features of the first instar larvae of Oxysarcodexia
(Diptera, Sarcophagidae) based on scanning electron microscope observations. Revista Brasileira
de Biologia, 46 (4), 741–746.
Lopes, H.S. & Leite, A.C.R. (1987) Third contribution to the knowledge of the Raviniini (Diptera,
Sarcophagidae), based on observations of the larvae, using scanning electron microscope.
Memórias do Instituto Oswaldo Cruz, 82 (3), 407–413.
Lopes, H.S. & Tibana, R. (1982) Sarcophagid flies (Diptera) from Sinop, State of Mato Grosso, Brazil.
Memórias do Instituto Oswaldo Cruz, 77 (3), 285–298.
Lopes, H.S. & Tibana, R. (1987) On Oxysarcodexia (Diptera, Sarcophagidae), with descriptions of five
new species, key, list and geographic distribution of the species. Revista Brasileira de Biologia,
47 (3), 329–347.
Lopes, H.S. & Tibana, R. (1991) Sarcophagidae (Diptera) de Roraima, Brasil. Acta Amazonica, 21,
151–157. [In Portuguese]
Lopes, H.S. (1933) Sobre algumas espécies de Sarcophaga do Brasil, com a descripção de cinco
espécies novas (Dipt. Sarcophagidae). Revista de Entomologia, 3 (2), 153–158. [In Portuguese]
Lopes, H.S. (1935) Notas sobre Sarcophagidae com descripção de tres espécies novas do Brasil (Dipt.).
Revista de Entomologia, 5, 315–322. [In Portuguese]
Lopes, H.S. (1938) Notas sobre Sarcophagidae neotrópicos. Um novo gênero e algumas novas
espécies. Memórias do Instituto Oswaldo Cruz, 33, 333–348 + 6 pls. [In Portuguese]
Lopes, H.S. (1939) Sobre alguns Sarcophagídeos de Misiones (Argentina). Physis, 17, 117–123. [In
Portuguese]
Lopes, H.S. (1943) Contribuição ao conhecimento das larvas dos Sarcophagidae com especial
referência ao esqueleto cefálico (Diptera). Memórias do Instituto Oswaldo Cruz, 38, 127–163. [In
Portuguese]

139
Lopes, H.S. (1946a) Um novo gênero e três novas espécies de “Sarcophagidae” do Brasil (Diptera).
Revista Brasileira de Biologia, 5 (4), 453–462. [In Portuguese]
Lopes, H.S. (1946b) Contribuição ao conhecimento das espécies do gênero Oxysarcodexia Towsend,
1917 (Diptera Sarcophagidae). Boletim Escolar Nacional de Vetores, 1, 62–134. [In Portuguese]
Lopes, H.S. (1946c) Sarcophagidae do México, capturados pelo professor A. Dampf. (Diptera).
Memórias do Instituto Oswaldo Cruz, 44 (1), 119–146. [In Portuguese]
Lopes, H.S. (1953) Seis novos “Sarcophagidae” neotrópicos (Diptera). Revista Brasileira de Biologia,
13, 41–51. [In Portuguese]
Lopes, H.S. (1969) A Catalogue of the Diptera of the Americas South of the United States: Family
Sarcophagidae. Departamento de Zoologia, Secretaria da Agricultura, 103, 88p.
Lopes, H.S. (1973a) Notes on some Neotropical Sarcophagidae studied by H. R. Dodge (Diptera).
Anais da Academia Brasileira de Ciências, 45, 293–299.
Lopes, H.S. (1973b) Collecting and rearing sarcophagid flies (Diptera) in Brazil, during 40 years.
Anais da Academia Brasileira de Ciências, 45, 279–291.
Lopes, H.S. (1975a) Sarcophagid flies Diptera from Pacatuba, State of Ceará, Brazil. Revista Brasileira
de Biologia, 34 (2), 271–294.
Lopes, H.S. (1975b) On female holotypes of some American species described by Francis Walker and
J. Macquart (Diptera, Sarcophagidae, Calliphoridae). Revista Brasileira de Biologia, 34 (2), 535–
550.
Lopes, H.S. (1975c) New or little known Oxysarcodexia (Diptera, Sarcophagidae). Revista Brasileira
de Entomologia, 35 (3), 461–483.
Lopes, H.S. (1975d) Bredin-Archbold Smithsonian Biological Survey of Dominica: The Sarcophagidae
of Dominica (Diptera). Anais da Academia Brasileira de Ciências, 45, 467–487.
Lopes, H.S. (1975e) On some new species of Sarcophagidae from Costa Rica (Diptera). Revista
Brasileira de Entomologia, 35 (3), 485–489.
Lopes, H. S. (1975f) Some new Sarcophagidae from Peru (Diptera). Revista Brasileira de Biologia, 34
(4), 573 – 580.
Lopes, H.S. (1976) On the holotypes, mostly females, of some Sarcophagidae (Diptera) described by
Francis Walker. Revista Brasileira de Biologia, 36, 629–641.
Lopes, H.S. (1978) On the Types of Some Neotropical Sarcophagidae described by H. R. Dodge
(Diptera). Revista Brasileira de Biologia, 38 (2), 501–507.

140
Lopes, H.S. (1980) On some Sarcophagidae (Diptera) from Pirapora, State of Minas Gerais, Brazil.
Revista Brasileira de Biologia, 40 (1), 5–8.
Lopes, H.S. (1985) On types of Sarcophagid flies (Diptera) described by Francis Walker, Camilo
Rondani and Van der Wulp. Revista Brasileira de Entomologia, 29 (3/4), 555–558.
Macquart, J. (1843) Diptères exotiques nouveaux ou peu connus. Tome deuxième, 3e partie. Memoires
de la Société Royale des sciences, de l’Agriculture et des Arts de Lille, 1842, 162–460. [In French]
Macquart, J. (1846) Diptéres exotiques nouveaux ou peu connus. Supplément. Memoires de la Société
Royale des sciences, de l’Agriculture et des Arts de Lille, 1844, 133–364 + pls. 1–20. [Last four
pages numbered 363, 364, 363, 364. Reprinted with pagination 5–238, Roret, Paris] [In French]
Macquart, J. (1851) Diptères exotiques nouveaux ou peu connus. Suite de 4.e supplément publié dans
les Mémoirs de 1849. Memoires de la Société Royale des sciences, de l’Agriculture et des Arts de
Lille, 1850, 134–294 + pls. 1–20. [In French]
Macquart, J. (1855) Diptères exotiques nouveaux ou peu connus. 5.e supplément. Memoires de la
Société Royale des sciences, de l’Agriculture et des Arts de Lille, 2 (1), 25–156 + pls. 1–7.
[Reprinted with pagination 5–136, Roret, Paris, 1855] [In French]
Marchiori, C.H. & Linhares, A.X. (1999) Constância, dominância e frequência de dípteros muscóides e
seus parasitóides (Hymenoptera e Coleoptera), associados a fezes frescas de bovinos, em
Uberlândia, MG. Anais da Sociedade de Entomologia do Brasil, 28, 275–387. [In Portuguese]
Marchiori, C.H. (2000) Parasitóides de Sarcophagula occidua Fabricius (Diptera, Sarcophagidae)
coletados em fezes bovinas em Goiás. Pesquisa Agropecuária Tropical, 30 (1), 17–21. [In
Portuguese]
Marchiori, C.H. (2007) Gnathopleura quadridentata Wharton, 1986 (Hymenoptera; Braconidae;
Alysiinae) and their hosts collected in different substrates in Caldas Novas, Goiás. Brazilian
Journal of Biology, 67 (1), 101–103.
Marchiori, C.H., Barbaresco, L.F. & Miranda, M.F. (2007a) Parasitóides de dípteros coletados em um
matadouro de Tupaciguara, Minas Gerais, Brasil. Semina: Ciências agrárias, 28 (4), 695–700. [In
Portuguese]
Marchiori, C.H., Filho, O.M.S., Borges, M.P. & Alvarenga, V.A. (2005) Ocorrência de Trybliographa
sp. (Hymenoptera: Figitidae: Eucoilinae) como parasitóide de Oxysarcodexia thornax (Walker,
1849) (Diptera: Sarcophagidae) no Brasil. Arquivos do Instituto de Biologia, 72 (2), 265–266. [In
Portuguese]
Marchiori, C.H., Filho, O.M.S., Borges, M.P. & Alvarenga, V.A. (2007b) Parasitóides de dípteros
coletados usando armadilha ptifall em Itumbiara, Goiás. Biotemas, 20 (1), 115–118. [In
Portuguese]

141
Marchiori, C.H., Oliveira, A.T. & Linhares, A.X. (2001) Artrópodes associados a massas fecais
bovinas no sul do estado de Goiás. Neotropical Entomology, 30 (1), 19–24. [In Portuguese]
Marchiori, C.H., Pereira, L.A., Filho, S.M.O. (2002) Parasitóides de Oxysarcodexia thornax (Walker,
1849) (Diptera: Sarcophagidae) coletados no estado de Goiás, Brasil. Revista de Patologia
Tropical, 31 (1), 134–137. [In Portuguese]
Marilius, J.C., Schnack, J.A., Mulieri, P.R. & Torretta, J.P. (2007) The Sarcophagidae (Diptera) of the
Coastline of Buenos Aires City, Argentina. Journal of the Kansas Entomological Society, 80 (3),
243–251.
Mattos, W.B. (1919) As Sarcophagas de São Paulo. Thesis, Faculdade de Medicina e Cirurgia de São
Paulo, iii + 116 + xii pp. [In Portuguese]
McAlpine, J.F. (1981) Morphology and terminology. In: McAlpine, J.F., Peterson, B.V., Shewell,
G.E., Teskey, H.J., Vockeroth, J.R. & Wood, D.M. (eds.), Manual of Nearctic Diptera. Vol. 1.
Monograph 27, Agriculture Canada, pp. 9–63.
Meiklejohn, K.A., Dowton, M. & Wallman, J.F. (2012) Notes on the distribution of 31 species of
Sarcophagidae (Diptera) in Australia, including new records in Australia for eight species.
Transactions of the Royal Society of South Australia, 136 (1), 56–64.
Mello-Patiu, C.A. & Pape, T. (2000) Definitions of Dexosarcophaga Townsend 1917 and
Sarcofahrtiopsis Hall 1933, including two new species and a redescription of Sarcofahrtiopsis
cuneata (Townsend 1935) (Diptera: Sarcophagidae). Boletín de Entomología Venezolana, 15 (2):
181–194.
Mendes, J. & Linhares, A.X. (1993) Sazonalidade, preferência por iscas e desenvolvimento ovariano
em várias espécies de Sarcophagidae (Diptera). Revista Brasileira de Entomologia, 37 (2), 355–
364. [In Portuguese]
Mendes, J. & Linhares, A.X. (2002) Cattle dung breeding Diptera in pastures in Southeastern Brazil:
diversity, abundance and seasonality. Memórias do Instituto Oswaldo Cruz, 97 (1): 37–41.
Moretti, T.C. & Godoy, W.A.C. (2013) Spatio-temporal dynamics and preference for type of bait in
necrophagous insects, particularly native and introduced blow flies (Diptera: Calliphoridae).
Journal of Medical Entomology, 50 (2), 415–424.
Moretti, T.C., Ribeiro, O.B., Thyssen, P.J. & Solis, D.R. (2008) Insects on decomposing carcasses of
small rodents in a secondary forest in Southeastern Brazil. European Journal of Entomology, 105,
691–696.
Moura, M.O. (2004) Variação especial como mecanismo promotor da coexistência em comunidades de
insetos necrófagos. Revista Brasileira de Zoologia, 21 (3), 409–419. [In Portuguese]

142
Moura, M.O., Carvalho, C.J.B. & Monteiro-Filho, E.L.A. (2005) Estrutura de comunidades necrófagas:
efeito da partilha de recursos na diversidade. Revista Brasileira de Zoologia, 22 (4), 1134–1140.
[In Portuguese]
Mulieri, P.R., Mariluis, J.C. & Patitucci, L.D. (2010) Review of the Sarcophaginae (Diptera:
Sarcophagidae) of Buenos Aires Province (Argentina), with a key and description of a new
species. Zootaxa, 2575, 1–37.
Mulieri, P.R., Patitucci, L.D., Schnack, J.A. & Mariluis, J.C. (2011) Diversity and seasonal dynamic of
an assemblage of Sarcophagid Diptera in a gradient of urbanization. Journal of Insect Science, 11
(91) 1–15.
Mulieri, P.R., Schnack, J.A., Mariluis, J.C. & Torretta, J.C. (2008) Flesh flies species (Diptera:
Sarcophagidae) from a grassland and a woodland in a nature reserve of Buenos Aires, Argentina.
Revista Biologia Tropical, 56 (3), 1287–1294.
Oliveira, T.C. & Vasconcelos, S.D. (2010) Insects (Diptera) associated with cadavers at the Institute of
Legal Medicine in Pernambuco, Brazil: implications for forensic entomology, Forensic Science
International, 198, 97–102.
Oliveira, V.C., D‟Almeira, J.M., Paes, M.J & Sanavria, A. (2002) Population dynamics of caliptrate
Diptera (Muscidade and Sarcophagidae) at the Rio-Zoo Foundantion, Rio de Janeiro, RJ, Brazil.
Brazilian Journal of Biology, 62 (2), 191–196.
Oliveira-Costa, J., Mello-Patiu C.A. & Lopes, S.M. (2001) Dípteros muscóides associados com
cadáveres humanos na cena da morte no estado do Rio de Janeiro - Brasil. Boletins do Museu
Nacional de Zoologia, 464, 1–6. [In Portuguese]
Pamplona, D., Maia, V.C., Couri, M.S., Lamas, C.J.E. & Aires, C.C.C. (2000) A survey of diptera on
Paquetá Island, Rio de Janeiro, Brazil. Entomologist’s Monthly Magazine, 136, 169–175.
Pape, T. & Dahlem, G.A. (2010) Sarcophagidae. In: Brown, B.V., Borkent, A., Cumming, J.M., Wood,
D.M., Woodley, N.E. & Zumbado, M. (eds), A Manual of Central American Diptera. Vol. 2. NRC
Research Press, Ottawa, pp. 1313–1335.
Pape, T. (1996) Catalogue of the Sarcophagidae of the world (Insecta: Diptera). Memoirs on
Entomology, International, 8, 1–558.
Pape, T. (2008) Nominal species of Australasian/Oceanian Sarcophagidae proposed by P. J. M.
Macquart (Insecta: Diptera). Zootaxa, 1702, 41–51.
Payne, J.A. & King, E.W. (1972) Insect succession and decomposition of pig carcasses in water.
Journal of the Georgia Entomological Society, 7 (3), 153–162.

143
Peckham, D.J. (1991) Consequences relating to the inclusion of female Sarcophagids (Diptera) as prey
of Oxybelus sparideus (Hymenoptera, Sphecidae). Annals of the Entomological Society of
America, 84 (2), 170–173.
Peckham, D.J., Kurczewski, F.F. & Peckham, D.B. (1973) Nesting behavior of Neartic species of
Oxybelus (Hymenoptera: Sphecidae). Annals of the Entomological Society of America, 66 (3),
647–661.
Pérez, S.P., Duque, P. & Wolff, M. (2005) Successional behavior and occurrence matrix of carrion-
associated arthropods in the urban area of Medellfn, Colombia. Journal of Forensic Sciences, 50
(2), 448–453.
Pombal, E.C.P. & Morellato, P.C. (2000) Differentiation of floral color and odor in two fly pollinated
species of Metrodorea (Rutaceae) from Brazil. Plant System Evolution, 221, 141–156.
Prado, A. & Fonseca, F. (1932) Algumas espécies novas de Sarcophagas da cidade de São Paulo
(Diptera. Stephanostomatidae). Revista Medico-Cirúrgica do Brasil, 40, 35–39. [In Portuguese]
Ramírez-Mora, M.A., Buenaventura, E., Gómez-P, L.M. & Amat, E. (2012) Uptadet cheklist and new
records of Calyptrate carrion flies (Diptera, Schizophora) from Valle de Aburrá and other
localities in Colombia. Entomotropica, 27 (1), 27–35.
Reed, H. B. (1958) A study of dog carcass communities in Tennessee, with special reference to the
insects. American Midland Naturalist, 59 (1), 213–245.
Reinhard, H.J. (1939) New genera and species of muscoid Diptera. Bulletin of Brooklyn Entomological
Society, 34: 61–74.
Roback, S.S. (1954) The evolution and taxonomy of the Sarcophaginae (Diptera, Sarcophagidae).
Illinois Biological Monographs, 23, 1–181. http://dx.doi.org/10.1086/401540
Rondani, C. (1850) Osservazioni sopra alquante specie di Esapodi ditteri del Museu Torinense. Nuovi
annali dele scienze naturali, 3 (2), 165–197. [In Italian]
Rosa, T.A., Babata, M.L.Y., Souza, C.M., Sousa, D., Mello-Patiu, C.A., Vaz-de-Mello, F.Z. &
Mendes, J. (2011) Arthropods associated with pig carrion in two vegetation profiles of Cerrado in
the State of Minas Gerais, Brazil. Revista Brasileira de Entomologia, 55 (3), 424–434.
Salazar-Ortega, J.A., Amat, E. & Gomez-Piñerez, L.M. (2012) A check list of necrophagous flies
(Diptera, Calyptrate) from urban area in Medellín, Colombia. Revista Mexicana de Biodiversidad,
83, 502–505.
Sánchez-Núñez, D.A. & Pineda-Mancera, J.E. (2012) Pollination and fruit set in the main neotropical
mangrove species from Southwestern Caribbean. Aquatic Botany, 103, 60–65.
Sanders, D.P. & Dorson, R.C. (1966) The insect complex associated with bovine manure in Indiana.
Annals of the Entomological Society of America, 59 (5), 955–959.

144
Schiner, I.R. (1868) Diptera. In: Reise der österreichischen Fregatte Novara um die Erde in den
Jahren 1857, 1858, 1859 unter den Befehlen des Commodore B, von Wüllerstorf-Urbair.
Zoologischer Theil. Vol. 01. Zweiter Band. Abtheilung, Wien, pp. 388–392. [In German]
Silva, K.P. & Mello-Patiu, C.A. (2008) Morfologia comparada da terminalia masculina de quatro
espécies de Oxysarcodexia Towsend, 1917 (Diptera, Sarcophagidae). Arquivos do Museu
Nacional, 66 (2), 363–372. [In Portuguese]
Soares, W.F. & Mello-Patiu, C.A. (2010) Two new Neotropical species of the genus Oxysarcodexia
Towsend (Diptera, Sarcophagidae). Revista Brasileira de Entomologia, 54 (1), 72–75.
Sousa, J.R.P., Esposito, M.C. & Carvalho-Filho, F.S. (2011) Composition, abundance and richness of
Sarcophagidae (Diptera: Oestroidea) in forests and forest gaps with different vegetation cover.
Neotropical Entomology, 40 (1), 20–27.
Stamper, T., Dahlem, G.A., Cookman, C., & Debry R.W. (2012) Phylogenetic relationships of flesh
flies in the subfamily Sarcophaginae based on three mtDNA fragments (Diptera: Sarcophagidae).
Systematic Entomology, 38 (1): 35–44. 10.1111/j.1365-3113.2012.00646.x
Tabor, K.L., Brewster, C.C. & Fell, R.D. (2004) Analysis of the successional patterns of insect on
carrion in Southwest Virginia. Journal of Medical Entomology, 41 (4), 785–795.
Tabor, K.L., Fell, R.D. & Brewster, C.C. (2005) Insect fauna visiting carrion in Southwst Virginia.
Forensic Science International, 150, 73–80.
Thomson, C.G. (1869) Diptera. Species nova descripsit. In: Kongliga Svenska Fregatten Eugenies resa
omkring jorden under befäl af C.A. Virgin, åren 1851–1853. Vetenskapliga Iakttagelser på H.M.
Konung Oscar den förstes befallning, af K. Svenska Vetenskaps-Akademien. Vol. 2. Zoologi. Part
1. Insecta. P.A. Norstedt & Soner, Stockholm, [1868], 617 pp. + 9 pls. [In German]
Tibana, R. & Mello, C.A. (1983a) Estudo sobre as fêmeas de Oxysarcodexia do grupo Peltata (Diptera,
Sarcophagidae). Revista Brasileira de Biologia, 43 (3), 241–250. [In Portuguese]
Tibana, R. & Mello, C.A. (1983b) Redescrição de Oxysarcodexia thornax (Walker, 1849) e descrição
de O. morretesi, sp. n. (Diptera, Sarcophagidae). Revista Brasileira de Entomologia, 27 (3), 277–
283. [In Portuguese]
Tibana, R. & Mello, C.A. (1985) O sintergito 6+7 nas fêmeas de Oxysarcodexia Towsend, 1917
(Diptera, Sarcophagidae). Revista Brasileira de Biologia, 45 (4), 439–445. [In Portuguese]
Towsend, C.H.T. (1917) Genera of the dipterous tribe Sarcophagini. Proceedings of the Biological
Society of Washington, 30, 189-198.
Vairo, K.P., Mello-Patiu, C.A. & Carvalho, C.J.B. (2011) Pictorial identification key for species of
Sarcophagidae (Diptera) of potential forensic importance in Southern Brazil. Revista Brasileira de
Entomologia, 55 (3), 333–347.

145
Vasconcelos, S.D. & Araujo, M.C.S. (2012) Necrophagous species of Diptera and Coleoptera in
northeastern Brazil: state of art and challenges for the Forensic Entomologist. Revista Brasileira
de Entomologia, 56 (1), 7–14.
Walker, F. (1836) Diptera. In: Curtis, J., Haliday, A. H. & Walker, F. (eds), Descriptions of insects
collected by Captain P. P. King, R. N., F. R. S., in the survey of the Straights of Magellan. Vol.
17. Trans. Linn. Soc. London, pp. 315–369.
Walker, F. (1849) Part IV. In: List of the specimens of dipterous insects in the collection of the British
Museum. Vol. 01. British Museum, London, pp. 689–1172.
Walker, F. (1853) Diptera. Part IV. In: Saunders, W.W. (ed.), Insecta Saundersiana: or characters of
undescribed insects in the collection of William Wilson Saunders, Esq., F.R.S., F.L.S., Vol. 1, Van
Voorst, London, 474 pp. + 8 pls.
Walker, F. (1857) Characters of undescribed Diptera in the collection of W. W. Saunders, Esq., F. R.
S. [part]. Transactions of the Entomological Society of London, London, 260–280 pp.
Walker, F. (1861) Characters of undescribed Diptera in the collection of W. W. Saunders, Esq., F. R.
S. [part]. Vol. 2. Transactions of the Entomological Society of London, London, 297–334 pp.
Walsh, G.C. & Posse, M.C. (2003) Abundance and seasonal distribution of predatory coprophilous
argentine rove beetles (Coleoptera: Staphylinidae), and their effects on dung breeding flies. The
Coleopterists Bulletin, 57 (1), 43–50.
Wharton, R.A. & Moon, R.D. (1979) Puparia of cyclorrhaphous Diptera from bovine dung in open
pasture and rangeland in the transition zone of Western North America. Annals of the
Entomological Society of America, 72 (1), 80–90.
Wiedemann, C.R.W. (1830) Aussereuropäische zweiflügelige Insekten. Als Fortsetzung des
Meigenschen Werkes. Zweiter Theil., Schulz, Hamm, xii + 684 pp. +5 pls. [In German]
Williston, S.W. (1896) On the Diptera of St. Vincent (West Indies). Transactions of the Entomological
Society of London, Part 3, 253–446. http://dx.doi.org/10.1111/j.1365-2311.1896.tb00965.x
Wolff, M., Uribe, A., Ortiz, A. & Duque, P. (2001) A preliminar study of forensic entomology in
Medellín, Colombia. Forensic Sicence International, 120, 53–59.
Wulp, F.M. (1895) Fam. Muscidae [part]. In: Godman, F. D. & Salvin, O. (eds). Biologia Centrali-
Americana. Vol. 2. Class Insecta. London, 289 pp.
Yepes-Gaurisas, D., Sánchez-Rodríguez, J.D., Mello-Patiu, C.A. & Echeverri, M.W. (2013)
Synantropy of Sarcophagidae (Diptera) in La Pintada, Antioquia-Colombia. Revista de Biologia
Tropical, 61 (3), 1275–1287.

146
Figure 1 – Genital structures of Oxysarcodexia Townsend, 1917 (Diptera: Sarcophagidae).
Abbreviations: b- basiphallus; d- distiphallus; C- cercus; Ep- epandrium; J- juxta; P- Phallus;
Po- postgonite; Pr- pregonite; Su- Surstylus; St- syntergosternite 7+8; V- vesica.

147
Appendices
Appendix 1 - Oxysarcodexia n. sp. 1. A) Habitus. B) Genitalia, lateral view. C) Genitalia,
anterior view. D) Genitalia, posterior view.

148
Appendix 2 - Oxysarcodexia n. sp. 2. A) Habitus. B) Genitalia, lateral view. C) Genitalia,
anterior view. D) Genitalia, posterior view.

149
Appendix 3 - Oxysarcodexia n. sp. 3. A) Habitus. B) Genitalia, lateral view. C) Genitalia,
anterior view. D) Genitalia, posterior view.

150
Appendix 4 - Oxysarcodexia n. sp. 4. A) Habitus. B) Genitalia, lateral view. C) Genitalia,
anterior view. D) Genitalia, posterior view.
A
B
C
D

151
Appendix 5 - Oxysarcodexia n. sp. 5. A) Habitus. B) Genitalia, lateral view. C) Genitalia,
anterior view. D) Genitalia, posterior view.
A
B
C D

152
Appendix 6 - Oxysarcodexia n. sp. 6. A) Habitus. B) Genitalia, lateral view. C) Genitalia,
anterior view. D) Genitalia, posterior view.

153
Appendix 7 – A) Oxysarcodexia admixta (Lopes, 1933); B) Oxysarcodexia adunca Lopes,
1975; C) Oxysarcodexia afficta (Wulp, 1895). Habitus; genitalia lateral view; genitalia
posterior view.
A
B
C

154
Appendix 8 – A) Oxysarcodexia amorosa (Schiner, 1868); B) Oxysarcodexia angrensis
(Lopes, 1933); C) Oxysarcodexia augusta Lopes, 1946. Habitus; genitalia lateral view;
genitalia posterior view.
A
A
B
C

155
Appendix 9 – A) Oxysarcodexia aura (Hall, 1937); B) Oxysarcodexia aurata (Macquart,
1851); C) Oxysarcodexia avuncula (Lopes, 1933). Habitus; genitalia lateral view; genitalia
posterior view.
A
C
B

156
Appendix 10 – A) Oxysarcodexia bakeri (Aldrich, 1916); B) Oxysarcodexia berlai Lopes,
1975; C) Oxysarcodexia bicolor Lopes, 1946. Habitus; genitalia lateral view; genitalia
posterior view.
A
B
C

157
Appendix 11 – A) Oxysarcodexia bikini Dodge, 1966; B) Oxysarcodexia carvalhoi Lopes,
1946; C) Oxysarcodexia chaetopygialis (Williston, 1896), lateral view with and without the
presence of the pregonite. Habitus; genitalia lateral view; genitalia posterior view.
A
C
B

158
Appendix 12 – A) Oxysarcodexia cingarus (Aldrich, 1916); B) Oxysarcodexia comparilis
(Reinhard, 1939); C) Oxysarcodexia conclausa (Walker, 1861). Habitus; genitalia lateral
view; genitalia posterior view.
A
C
B

159
Appendix 13 – A) Oxysarcodexia confusa Lopes, 1946; B) Oxysarcodexia corolla Dodge,
1965, presenting the genitalia in lateral view, adapted from Lopes 1978; C) Oxysarcodexia
cuernavaca Dodge, 1966, presenting the genitalia in lateral and posterior views, adapted from
Dodge, 1966.
A
C
B

160
Appendix 14 – A) Oxysarcodexia culmiforceps (Dodge, 1966); B) Oxysarcodexia culminata
(Aldrich, 1916), both presenting the habitus; genitalia in lateral view; and genitalia in posterior
view; C) Oxysarcodexia cyanea Lopes, 1975, presenting the genitalia in lateral view, adapted
from Lopes 1975b.
B
C
A

161
Appendix 15 – A) Oxysarcodexia cyaniforceps (Hall, 1933), presenting the genitalia in lateral
view, adapted from Lopes 1975c; B) Oxysarcodexia diana (Lopes, 1933); B) Oxysarcodexia
eberti Lopes & Tibana, 1987. Habitus; genitalia lateral view; genitalia posterior view.
A
C
B

162
Appendix 16 – A) Oxysarcodexia edwardsi Lopes, 1946; B) Oxysarcodexia favorabilis
(Lopes, 1935) with habitus; genitalia lateral view; genitalia posterior view, and, for O.
favorabilis, also anterior view; C) Oxysarcodexia festiva Lopes & Tibana, 1987, presenting
the genitalia in lateral view and cerci in posterior view , adapted from Lopes & Tibana 19875.
A
B
C

163
Appendix 17 – A) Oxysarcodexia flavipes Lopes & Tibana, 1987; B) Oxysarcodexia floricola
Lopes, 1975; C) Oxysarcodexia fluminensis Lopes, 1946. Habitus; genitalia lateral view;
genitalia posterior view.
A
B
C

164
Appendix 18 – A) Oxysarcodexia fraterna (Lopes, 1946); B) Oxysarcodexia fringidea
(Curran & Walley, 1934); B) Oxysarcodexia galeata (Aldrich, 1916). Habitus; genitalia lateral
view; genitalia posterior view, except for O. fraterna which is presented only lateral view.
A
B
C

165
Appendix 19 – A) Oxysarcodexia grandis Lopes, 1946; B) Oxysarcodexia inflata Lopes,
1975; C) Oxysarcodexia injuncta (Walker, 1858). Habitus (except for O. grandis); genitalia
lateral view; genitalia posterior view.
A
C
B

166
Appendix 20 – A) Oxysarcodexia insolita Lopes, 1946; B) Oxysarcodexia intona (Curran &
Walley, 1934); C) Oxysarcodexia jamesi Dodge, 1968. Habitus; genitalia lateral view;
genitalia posterior view.
A
C
B

167
Appendix 21 – A) Oxysarcodexia major Lopes, 1946; B) Oxysarcodexia marina (Hall, 1938);
C) Oxysarcodexia meridionalis (Engel, 1931). Habitus; genitalia lateral view; genitalia
posterior view.
A
C
B

168
Appendix 22 – A) Oxysarcodexia mitifica Lopes, 1953; B) Oxysarcodexia modesta Lopes,
1946; C) Oxysarcodexia molitor (Curran & Walley, 1934). Habitus; genitalia lateral view;
genitalia posterior view, except for O. molitor with only lateral view, adapted from Lopes
1946f.
A
C
B

169
Appendix 23 – A) Oxysarcodexia morretesi Tibana & Mello, 1983; B) Oxysarcodexia nitida
Soares & Mello-Patiu, 2010; B) Oxysarcodexia notata Soares & Mello-Patiu, 2010. Habitus;
genitalia lateral view; genitalia posterior view.
A
C
B

170
Appendix 24 – A) Oxysarcodexia occulta Lopes, 1946; B) Oxysarcodexia orbitalis Dodge,
1966, adopted from Dodge, 1966; C) Oxysarcodexia pallisteri Dodge, 1966. Habitus, except
for O. orbitalis; genitalia lateral view; genitalia posterior view.
A
C
B

171
Appendix 25 – A) Oxysarcodexia parva Lopes, 1946; B) Oxysarcodexia paulistanensis
(Mattos, 1919); C) Oxysarcodexia peculiaris Lopes, 1975. Habitus; genitalia lateral view;
genitalia posterior view.
A
C
B

172
Appendix 26 – A) Oxysarcodexia peltata (Aldrich, 1916); B) Oxysarcodexia perneta
(Walker, 1861); C) Oxysarcodexia peruviana (Lopes, 1975), with only genitalia lateral view,
adapted from Lopes 1975f. Habitus; genitalia lateral view; genitalia posterior view.
A
B
C

173
Appendix 27 – A) Oxysarcodexia petropolitana Lopes, 1975; B) Oxysarcodexia plebeja
Lopes, 1946; C) Oxysarcodexia ramosa (Reinhard, 1939), adapted from Dodge 1966. Habitus,
except for O. ramosa; genitalia lateral view; genitalia posterior view.
A
B
C

174
Appendix 28 – A) Oxysarcodexia riograndensis Lopes, 1946; B) Oxysarcodexia sarcinata
Lopes, 1953; C) Oxysarcodexia similata Lopes & Tibana, 1987. Habitus, except for O.
ramosa; genitalia lateral view; genitalia posterior view.
A
B
C

175
Appendix 29 – A) Oxysarcodexia simplicoides (Lopes, 1933); B) Oxysarcodexia terminalis
(Wiedemann, 1830); C) Oxysarcodexia thornax (Walker, 1849). Habitus; genitalia lateral
view; genitalia posterior view.
A
B
C

176
Appendix 30 – A) Oxysarcodexia timida (Aldrich, 1916); B) Oxysarcodexia trivialis (Wulp,
1895); C) Oxysarcodexia varia (Walker, 1836). Habitus; genitalia lateral view; genitalia
posterior view, and also for O. varia, genitalia anterior view.
A
B
C

177
Appendix 31 – A) Oxysarcodexia ventricosa (Wulp, 1895); B) Oxysarcodexia villosa Lopes,
1946; C) Oxysarcodexia vittata (Walker, 1836). Habitus, except for O. vittata; genitalia lateral
view; genitalia posterior view, and also, for O. villosa genitalia in anterior view.
A
C
B

178
Appendix 32 – A) Oxysarcodexia wygodzinskyi Lopes & Tibana, 1987; B) Oxysarcodexia
xannthosoma (Aldrich, 1916); C) Oxysarcodexia xon (Dodge, 1968), adapted from Lopes
1975c; D) Oxysarcodexia zayasi Dodge, 1956. Habitus; genitalia lateral view; genitalia
posterior view. For O. xon only genitalia in lateral view.
A
B
D
C

179
6. CAPÍTULO II
ABORDAGEM FILOGENÉTICA DO GÊNERO Oxysarcodexia (DIPTERA: SARCOPHAGIDAE) COM
ESPECIAL REFERÊNCIA À GENITÁLIA ASSIMÉTRICA DE ALGUNS MACHOS
CARINA MARA DE SOUZA1, DAVID K-B CHEUNG
2, THOMAS PAPE
3 & PATRICIA
JACQUELINE THYSSEN4
1 Departamento de Biologia Animal, Universidade de Campinas - UNICAMP, CP: 6109,
CEP:13083-970, Campinas, São Paulo, Brasil. E-mail: [email protected]
2 Museu de História Natural da Dinamarca, Universitetsparken 15, DK - 2100 Copenhagen,
Dinamarca. E-mail: [email protected]
3 Museu de História Natural da Dinamarca, Universitetsparken 15, DK - 2100 Copenhagen,
Dinamarca. E-mail: [email protected]
4 Departamento de Microbiologia e Biologia, Universidade Federal de Pelotas - UFPel, CP:
354, CEP: 96010-900, Pelotas, Rio Grande do Sul, Brasil. E-mail: [email protected]
RESUMO
Uma análise filogenética baseada em caracteres morfológicos a partir do exame
machos de 54 espécies de Oxysarcodexia Townsed, 1917 (Diptera: Sarcophagidae) foi
realizada a fim de investigar a assimetria fálica vista em algumas espécies e a ocorrência de
possíveis agrupamentos de espécies dentro do gênero. Para isso, foram realizadas análises de
parcimônia por busca heurística, utilizando as metodologias de busca tradicional e novas
tecnologias do programa TNT, com e sem pesos implicados e valores de suporte de ramos de
Bremer, tanto para a matriz completa quanto para a matriz contendo apenas caracteres da
terminália. Além disso, micrografias rotacionais foram produzidas, após a confecção de
imagens de microscopia eletrônica de varredura para Oxysarcodexia fringidea (Curran &
Walley, 1934), Oxysarcodexia timida (Aldrich, 1916) e Oxysarcodexia varia (Walker, 1836),
utilizadas como modelos, para analisar mais pormenorizadamente as alterações morfológicas
consequentes da assimetria. Foram listados 73 caracteres morfológicos, dos quais 14 foram
referentes a caracteres não localizados na terminália e 59 exclusivos da terminália masculina.
A assimetria detectada foi do tipo direcional sinistral, presente em oito espécies, restringindo-

180
se aos lobos terminais da vésica. Esse estado de caráter foi considerado homoplásico, com
base nas topologias obtidas. Quanto aos agrupamentos de espécies, a topologia encontrada
mostrou similaridade com o proposto na literatura, embora sem completa correspondência
para as relações entre as espécies, exceto para o “grupo ventricosa”, para o qual não houve
nenhuma correspondência.
Palavras-chave: Cladística, caracteres morfológicos, moscas coprófagas, microscopia
eletrônica de varredura rotacional.
INTRODUÇÃO
A reconstrução da filogenia de muitos grupos da ordem Diptera, incluindo aqueles
pertencentes à família Sarcophagidae (Arthropoda: Diptera), pode ser satisfatoriamente
acessada por meio do estudo de caracteres morfológicos, especialmente daqueles presentes na
terminália dos machos (e.g. Pape, 1992; 1996; Blackith et al., 1997; Giroux e Wheeler, 2009;
Giroux et al., 2010; Whitmore et al., 2013). Análises de sequências moleculares baseadas em
marcadores mitocondriais como citocromo oxidase subunidade I, 12S, 16S, e citocromo b,
e/ou nucleares como RNA ribossomal 18S e 28S, fator de elongação 1-α e fragmentos do gene
CAD, vão de encontro às abordagens morfológicas (e.g. Wells et al. 2001; Kutty et al., 2008),
corroborando entre si a confiabilidade do resgate filogenético baseado em ambas as fontes de
dados.
A morfologia externa dos sarcofagídeos é considerada, de maneira geral, altamente
homoplásica (Giroux et al., 2010). Contudo, a terminália dos machos é uma importante fonte
de dados filogenéticos, por apresentar estruturas com elevado grau de complexidade, podendo
assim ser utilizada para esclarecer a história filogenética de um determinado grupo, a partir de
análises minuciosas e comparativas (Blackith et al., 1997; Giroux et al., 2010; Whitmore et
al., 2013).
Agrupamentos para algumas espécies do gênero Oxysarcodexia Townsend, 1917
(Diptera: Sarcophagidae) já foram propostos na literatura como detalhado abaixo:
“grupo paulistanensis” (Lopes, 1973; 1975b), incluindo Oxysarcodexia bikini Dodge, 1966,
Oxysarcodexia injuncta (Walker, 1857), Oxysarcodexia paulistanensis Prado & Fonseca, 1932
e Oxysarcodexia riograndensis Lopes, 1946;

181
“grupo peltata” (Lopes, 1975a; Tibana & Mello, 1983), incluindo Oxysarcodexia aurata
(Macquart, 1851), Oxysarcodexia culminata (Aldrich, 1916), Oxysarcodexia culmiforceps
Dodge, 1966, Oxysarcodexia fringidea (Curran & Walley, 1934), Oxysarcodexia intona
(Curran & Walley, 1934) e Oxysarcodexia peltata (Aldrich, 1916);
“grupo thornax” (Lopes, 1976), incluindo Oxysarcodexia afficta (Wulp, 1895),
Oxysarcodexia conclausa (Walker, 1861), Oxysarcodexia thornax (Walker, 1849) e
Oxysarcodexia timida (Aldrich, 1916);
“grupo ventricosa” (Lopes, 1975a), incluindo Oxysarcodexia admixta (Lopes, 1933),
Oxysarcodexia avuncula (Lopes, 1933), Oxysarcodexia berlai Lopes, 1975, Oxysarcodexia
carvalhoi Lopes, 1946, Oxysarcodexia diana (Lopes, 1933) e Oxysarcodexia ventricosa
(Wulp, 1895);
“grupo Xarcophaga” (Lopes, 1975a; Soares & Mello-Patiu, 2010), incluindo
Oxysarcodexia favorabilis (Lopes, 1935), Oxysarcodexia fraterna (Lopes, 1946),
Oxysarcodexia nitida Soares & Mello-Patiu, 2010, Oxysarcodexia notata Soares & Mello-
Patiu, 2010, Oxysarcodexia pallisteri Dodge, 1966, Oxysarcodexia peruviana (Lopes, 1973),
Oxysarcodexia vittata (Lopes, 1946) e Oxysarcodexia xon (Dodge, 1968).
Esses grupos foram baseados em caracteres morfológicos externos de machos e/ou de
fêmeas; entretanto, nenhuma abordagem filogenética formal para as relações interespecíficas
foi proposta.
Apesar da homogeneidade na morfologia externa das espécies inclusas em
Oxysarcodexia, é possível observar diversas modificações nas estruturas fálicas,
especialmente na vésica. Em um número considerável de espécies, os ramos da vésica sofrem
“deformações”, tornando-se assimétricos (Souza et al. in prep.). Uma vez que os organismos
do filo Arthropoda apresentam como via de regra a simetria bilateral (Triplehorn & Johnson,
2005), a ocorrência de assimetria torna-se uma característica marcante e instigante. Em
Insecta, a assimetria já foi relatada para Orthoptera, nas linhas laterais que cobrem a asa; para
Thysanoptera, na lateral do estilete mandibular; para Coleoptera, no entalhe lateral presente na
mandíbula; dentre outros insetos, cuja assimetria é observada na genitália dos machos. Os
traços assimétricos de um mesmo grupo podem ser dextrais (deslocados para a direita),
sinistrais (deslocados para a esquerda) ou randômicos (ambas as formas comuns e em
frequências similares) (Palmer, 2009).

182
A assimetria de estruturas morfológicas nos animais, assim como nos protistas e nas
plantas, é considerada um traço extremamente raro que corresponde a uma característica que
evoluiu por várias vezes de forma independente e convergente (Huber et al., 2007; Palmer,
2009). Assim, a partir de uma filogenia baseada em caracteres morfológicos e da análise
ultraestrutral por meio de microscopia eletrônica de varredura das estruturas fálicas de
algumas espécies, a ocorrência de assimetria em Oxysarcodexia foi investigada numa tentativa
de rastrear o surgimento desta característica dentro deste gênero. Além disso, buscou-se
elucidar a relação entre as espécies de Oxysarcodexia com base na filogenia morfológica
apresentada.
MATERIAL E MÉTODOS
O material utilizado para a confecção de uma matriz de caracteres morfológicos
baseada em machos compreendeu 54 espécies de Oxysarcodexia (Apêndice I) disponíveis para
exame. Fêmeas e larvas não foram inclusas nas análises devido à dificuldade de identificação
em nível específico, ao não conhecimento dessas formas para muitas espécies e também, para
as conhecidas, à indisponibilidade de espécimes para estudo. De acordo com uma análise
filogenética prévia para Sarcophaginae (Giroux et al., 2010), as espécies Dexosarcophaga
transita (Townsend, 1917) e Ravinia belforti (Prado & Fonseca, 1932) foram inclusas nas
análises como grupo externo, por pertencerem a gêneros considerados filogeneticamente
próximos à Oxysarcodexia.
A matriz de dados foi compilada usando o programa Mesquite versão 2.75 (Maddison
& Maddison, 2011), incluindo caracteres morfológicos registrados a partir do exame
comparativo entre as espécies. Para os caracteres morfológicos não localizados na terminália,
a terminologia usada seguiu McAlpine (1981) e, para aqueles presente na terminália dos
machos, seguiu-se Mello-Patiu & Pape (2000) e Giroux et al. (2010). A filogenia foi inferida
com auxílio do programa TNT (Goloboff et al., 2008), a partir de análises de parcimônia por
busca heurística, utilizando as metodologias de busca tradicional (“traditional search” com o
algoritmo heurístico “tree-bissection-reconnection” – TBR; RAM 1.000 Mb; memória de
99.999 árvores; “random seed” de 50.000 árvores, 1.000 réplicas; e 1.000 árvores salvas por
replicação) e novas tecnologias de busca (“new technology search” com o algoritmo heurístico
TBR; RAM 1.000 Mb; memória de 99.999 árvores; “ratched search” com 10 sequências

183
adicionadas inicialmente e 10 árvores salvas por replicação). As árvores mais parcimoniosas
foram geradas considerando-se pesos iguais e pesos implicados (k=3). Os valores de suporte
de Bremer para os ramos foram calculados para 50.000 árvores sub-ótimas com até 10 passos
a mais que a árvore mais curta obtida. Foi realizada também uma análise por busca tradicional
e valores de suporte de Bremer (mesmos parâmetros descritos acima) para a matriz
considerando apenas caracteres da terminália, considerados na literatura como mais
informativos.
Para analisar as alterações morfológicas consequentes da assimetria, as espécies
Oxysarcodexia fringidea (Curran & Walley, 1934), Oxysarcodexia timida (Aldrich, 1916) e
Oxysarcodexia varia (Walker, 1836), que apresentam vésicas assimétricas e são mais
frequentes que as demais portadoras de tal característica, foram utilizadas como modelos de
estudo. Para isso, essas espécies foram submetidas à técnica de microscopia eletrônica de
varredura (MEV) para a produção de micrografias rotacionais. A terminália de espécimes
alfinetados foi cuidadosamente seccionada no nível do epândrio, colocada em ácido lático
80% aquecido por aproximadamente 5 minutos, dissecada, lavada em água morna e
desidratada em etanol 96%. O processo de clarificação com ácido lático foi preferido ao
hidróxido de potássio a fim de evitar deformações em estruturas membranosas do falo
(Triplehorn & Johnson, 2005). A confecção de micrografias rotacionais interativas foi
realizada de acordo com a metodologia proposta por Cheung et al. (2013). Cada falo, após
secar naturalmente, foi posicionado em um suporte metálico (stub) com fita adesiva dupla
face, coberto com platina para que, então, as imagens fossem produzidas, sequencialmente,
utilizando um microscópio eletrônico de varredura do tipo “JEOL-JSM-6335F”, localizado no
Museu de Zoologia da Universidade de Copenhagen, Copenhagen, Dinamarca. As imagens
foram submetidas à edição para ajuste de exposição, brilho e contraste, além do ajuste de
tamanho e qualidade, para assegurar que todas as imagens ficassem alinhadas possibilitando
uma transição contínua quando em sequência. As micrografias foram então agrupadas em uma
animação rotacional, disponibilizada na web com o auxílio de um plug-in específico (Magic
360®
).
RESULTADOS
Foram listados 73 caracteres morfológicos, dentre os quais 30 referem-se a caracteres

184
multiestado e 43 a binários. Do total, foram 26 caracteres localizados não na terminália,
distribuídos na cabeça, tórax e abdômen, excluindo a terminália, e 47 restritos à terminália dos
machos, divididos entre sintergosternito 7+8, surstilo, cercos, pênis, pré e pós-gonitos e vésica
(Apêndices II e III). As árvores de consenso resultantes de 12 árvores mais parcimoniosas
geradas pela método de parcimônia e busca heurística inferida por busca tradicional (Fig. 1) e
de três árvores mais parcimoniosas geradas pelas novas tecnologias de busca (Fig. 2),
considerando pesos iguais para os caracteres em ambos os casos, apresentaram as mesmas
ramificações e topologias, com exceção apenas de O. mitifica. As árvores de consenso, quando
associadas ao valor de suporte de Bremer (Figs. 3 e 4), apresentaram as mesmas topologias e
valores associados, exceto para o clado O. aurata, O. intona e O. peltata, melhor acomodado
na árvore resultante da busca por novas tecnologias. A utilização do peso implicado resultou
em árvores com as mesmas ramificações e topologias, tanto para a busca tradicional (Fig. 5)
quanto via novas tecnologias de busca (Fig. 6). A análise sob os critérios de busca tradicional,
pesos iguais e suporte de Bremer, utilizando a matriz com caracteres apenas da terminália,
gerou uma árvore de consenso a partir de 14 árvores mais parcimoniosas (Fig. 7). Sob os
mesmos critérios, porém com pesos implicados, observou-se maior separação entre os clados,
apesar do suporte de Bremer ainda ser considerado baixo (Fig. 8).
A genitália assimétrica foi detectada em oito espécies: Oxysarcodexia corolla Dodge,
1965, Oxysarcodexia edwardsi Lopes, 1946, Oxysarcodexia fringidea, Oxysarcodexia ramosa
(Reinhard, 1939), Oxysarcodexia sarcinata Lopes, 1953, Oxysarcodexia timida,
Oxysarcodexia varia e Oxysarcodexia villosa Lopes, 1946 (Fig. 9), sendo que exemplares de ,
O. edwardsi, O. fringidea, O. sarcinata, O. timida, O. varia e O. villosa foram examinados e
O. corolla e O. ramosa foram determinadas assimétricas com base nas descrições originais e
suas ilustrações (Lopes, 1946; Dodge, 1965). As micrografias rotacionais dos falos de O.
fringidea, O. timida e O. varia foram disponibilizadas no sítio eletrônico
<http://dkbdigitaldesigns.com/semrotate/sem.html>. A análise por MEV da composição fálica
das espécies O. fringidea, O. timida e O. varia (Fig. 10) permitiu verificar que a assimetria
está restrita aos lobos terminais da vésica, com redução e/ou deslocamento do lobo terminal
esquerdo, correspondendo a uma assimetria direcional sinistral.

185
DISCUSSÃO
Os baixos valores de suporte de Bremer alcançados em todas as análises refletem o alto
grau de homoplasia da matriz de caracteres. Isso pode ser em parte decorrente de uma
fragilidade da matriz em resposta à escolha dos caracteres, mas é mais provável que seja
devido ao alto grau de homoplasia já relatado na literatura para a morfologia externa de
Sarcophagidae, assim como de outros dípteros (e.g. Couri & Pont, 2000; O‟Hara, 2002;
Giroux et al., 2010). Apesar das árvores terem sido geradas a partir da matriz com caracteres
homoplásicos, a resolução entre os taxa parece ser razoável.
Um agrupamento inserido em Sarcophaginae e denominado “clado Ravinia”
compreende os gêneros Ravinia Robineau-Desvoidy, 1863 e Oxysarcodexia Townsend, 1917,
considerados grupos próximos ou irmãos em várias filogenias. A presença de ornamentação,
representada por estrias transversais (“festoon-like”) largas e nítidas em torno do anel da
cavidade oral das larvas de primeiro estádio e a presença de ctenídios formados por espinhos
achatados no fêmur médio, são características que corroboram a proximidade destes gêneros
(Roback, 1954; Downes, 1955; Lopes, 1982; Pape, 1996; Giroux et al., 2010). As filogenias
baseadas em caracteres moleculares, no entanto, são divergentes. Stamper et al. (2012),
utilizando análises Bayesianas para avaliar a variação gênica proveniente das regiões
mitocondriais COI, a subunidade II (COII) e a subunidade 4 da desidrogenase (ND4),
encontrou suporte para o clado Ravinia + Oxysarcodexia, embora mais consistente somente
após considerar previamente a politomia. Já Kutty et al. (2010) não encontraram suporte para
uma relação próxima destes dois gêneros ao analisar regiões gênicas mitocondriais (12S, 16S,
COI e citocromo b) e nucleares (18S, 28S, a região carbamoil fosfato sintetase – CAD
(rudimentar) e o fator de elongação 1-α). Vale ressaltar que no estudo de Kutty et al. (2010)
apenas uma espécie de cada gênero foi inclusa nas análises, o que pode ter gerado um viés nos
resultados, culminando na ausência de suporte para a relação entre esses gêneros. Nas
presentes análises, Ravinia enraizou o cladograma, tendo como grupo-irmão um clado
formado por Dexosarcophaga Townsend, 1917 e Oxysarcodexia. Essa divergência na
proximidade desses gêneros, em comparação à literatura, pode ter ocorrido devido à inclusão
de apenas uma espécie de cada um desses gêneros, enviesando a filogenia aqui obtida.
A formação de um clado para as espécies O. nitida, O. notata e O. vittata sob a análise
por busca tradicional com pesos implicados para a matriz com caracteres apenas da terminália

186
corrobora a proximidade destas espécies já apontada por Lopes (1975a) e Soares & Mello-
Patiu (2010). Esses autores nomeiam o grupo formado por tais espécies como “grupo
Xarcophaga”, baseando o agrupamento na forma do falo, alongado e afinado, com um
alargamento apical característico.
As espécies O. injuncta, O. paulistanensis e O. riograndensis, pertencentes ao “grupo
paulistanensis”, caracterizado pela morfologia das fêmeas, com o tergito 8 presente e a
ausência de cerdas representando o tergito anal (Lopes, 1975b), não formaram um clado único,
considerando a árvore recuperada a partir da análise por busca tradicional com pesos
implicados para a matriz com caracteres apenas da terminália. Do “grupo paulistanensis”,
apenas as espécies O. paulistanensis e O. riograndensis se apresentaram como grupos irmãos,
abrigados em um único clado. As demais espécies desse grupo ficaram agrupadas em um
clado maior, que abrigou também outras espécies que apresentam a vésica com o ápice
filamentoso, como visto, por exemplo, em O. carvalhoi e O. amorosa.
Apesar de O. intona e O. aurata formarem um grupo irmão de O. peltata em todas as
árvores geradas, as demais espécies do “grupo peltata” (O. culminata, O. culmiforceps e O.
fringidea), caracterizado pela redução do tergito genital nas fêmeas (Lopes 1975c), não se
apresentaram como irmãs desse clado. Da mesma forma, a dilatação dorsoapical larga do falo
se mostrou como uma característica homoplásica, uma vez que O. galeata e O. varia, que
também apresentam tal estado de caráter, foram acomodadas em clados filogeneticamente
distantes.
O “grupo thornax”, caracterizado pela presença, nas fêmeas, de uma larga região
membranosa entre o tergito 5 e o tergito 6+7, sendo este último reduzido, apresentou-se nas
presentes análises formando um clado que não incluiu O. timida e foi filogeneticamente
próximo a O. occulta. Similarmente ao observado para o “grupo peltata”, a dilatação
dorsoapical média do falo se mostrou como uma característica homoplásica devido à formação
dos clados para O. conclausa, O. riograndensis, O. thornax e O. vittata.
Para as espécies do “grupo ventricosa”, caracterizado pela presença, nas fêmeas, do
tergito 6+7 inteiro e fortemente esclerotizado (Lopes, 1975a), não foi observada a formação de
agrupamentos em nenhuma das árvores obtidas. Isso pode ter ocorrido devido à ausência de
caracteres da morfologia das fêmeas na matriz utilizada e responsáveis pelo agrupamento
proposto na literatura.

187
Em abordagens filogenéticas, a simetria bilateral é considerada um estado padrão
devido à predefinição dos eixos anteroposterior e dorsoventral. As alterações no padrão
simétrico surgem devido a mudanças nos eixos que deslocam o lado oposto, embora os
mecanismos envolvidos sejam ainda desconhecidos para a maioria dos organismos que
apresentam estruturas morfológicas assimétricas. Os caracteres assimétricos podem ser
divididos em dois tipos: antissimétrico ou assimetria aleatória, no qual as formas sinistrais
(deslocamento para a esquerda) e dextrais (deslocamento para a direita) são igualmente
comuns numa mesma espécie; e assimetria direcional, no qual a maioria dos indivíduos
apresenta assimetria no mesmo lado (Palmer, 2004). As assimetrias direcionais fixas permitem
o desenvolvimento de estudos moleculares a fim de investigar se a regulação gênica dos
processos envolvidos na assimetria é feita por alguns ou vários genes. Para assimetrias
aleatórias, a investigação de regulação gênica é praticamente impossível devido à variação
dextral ou sinistral ser considerada uma variação fenotípica quase sempre sem uma base
genética (Palmer, 2009). Além de aranhas (Pholcidae e Theridiidae), Apterygota, Paleoptera,
Orthoptera, Phasmida, Embiidina, Grylloblattodea, Mantophasmatodea, Plecoptera,
Dermaptera, Zoraptera, Dictyoptera, Thysanoptera, Heteroptera, Psocodea, Neuropterida,
Siphonaptera, Mecoptera, Strepsiptera, Coleoptera, Hymenoptera, Trichoptera e Lepidoptera
possuem representantes portadores de caracteres assimétricos já relatados na literatura (Huber
et al., 2007).
Das espécies de Oxysarcodexia com vésica assimétrica, cinco (O. corolla, O. edwardsi,
O. fringidea, O. timida e O. villosa) ocorrem exclusivamente na região Neotropical; uma (O.
ramosa) é exclusiva da região Neártica; uma (O. sarcinata) ocorre nas regiões Neotropical e
Neártica; e outra (O. varia) nas regiões Neotropical e Australásia/Oceânica (Souza et al., in
prep.). A ocorrência de assimetria fálica em Oxysarcodexia já foi associada a deformações
causadas por compressões durante a fase de pupa (Lopes, 1946). Contudo, esta justificativa
parece ser procedente apenas para variações fenotípicas sem conexão com fatores genéticos, o
que parece não ser o caso da genitália assimétrica das espécies anteriormente mencionadas.
Isto porque, no caso de ser fixa (quando o desvio ou modificação da estrutura sempre ocorre
do mesmo lado), a assimetria pode ser considerada independente de fatores extrínsecos, como
compressão, e dependente de fatores genéticos, logo um caráter herdado (Palmer, 2009).
O tipo de assimetria visto em Oxysarcodexia, direcional sinistral com redução e/ou

188
deslocamento do lobo terminal, é similar ao observado em Syrphidae, que geralmente
apresenta deslocamento ou encurtamento das estruturas genitais do lado esquerdo (Metcalf,
1921), embora neste caso a assimetria possa ser decorrente da rotação que as estruturass da
genitália sofrem após a emergência do adulto. Em Tachydromiinae (Diptera: Empididae), por
exemplo, a terminália assimétrica pode ser encontrada em todos os taxa, sendo que o lado
direito apresenta-se mais alargado que o esquerdo (Chvála, 1975; Cumming & Cooper, 1992),
contrapondo o visto em Oxysarcodexia.
A ocorrência de genitália assimétrica em Diptera é considerada esporádica, embora em
Acalyptratae o ápice assimétrico do falo seja recorrente (Huber et al., 2007). Na superfamília
Empidoidea e em Phoridae, por exemplo, considera-se que todos os machos apresentam
genitálias assimétricas em certo grau, apesar da simetria bilateral ser considerada o estado
padrão (Huber et al., 2007; Nakayama, 2008; 2012). Huber et al. (2007) apresentam um
panorama amplo e detalhado sobre a ocorrência de assimetria entre os dípteros e outros
artrópodes, listando a presença desse estado para algumas espécies de Agromyzidae,
Anthomyiidae, Asteiidae, Atelestidae, Bombyliidae, Brachystomatidae, Ceratopogonidae,
Diopsidae, Dolichopodidae, Drosophilidae, Dryomyzidae, Empididae, Ephydridae, Hybotidae,
Lauxaniidae, Lonchaeidae, Micropezidae, Mycetophilidae, Periscelididae, Phoridae,
Pipunculidae, Scatopsidae, Sepsidae, Sphaeroceridae, Syrphidae, Tachinidae, Tephritidae,
Thaumaleidae, Tipulidae (Diptera: Arthropoda). As estruturas morfológicas da terminália dos
machos que podem ser acometidas pela assimetria são os pós e pré-gonitos, o surstilo, o
epândrio, o hipândrio, os gonostilos, os cercos e o falo (Huber et al., 2007).
A análise das árvores recuperadas com base em caracteres morfológicos indica que a
assimetria na genitália de Oxysarcodexia possa ser um caráter homoplásico, que surgiu várias
vezes e de maneira independente no grupo. Situação semelhante ocorre em Phoridae e
Pipunculidae, cujos caracteres assimétricos da genitália dos machos parecem ter evoluído
várias vezes dentro da família, sendo filogeneticamente informativos para o estudo cladístico
de determinados grupos, gêneros ou mesmo subfamílias (Rafael & De Meyer, 1992;
Nakayama, 2012).
Várias hipóteses são relatadas na literatura na tentativa de esclarecer a evolução da
assimetria em estruturas genitais. Dentre elas, as pressões espaciais causadas pela disposição
das estruturas internas (favorecendo a fixação assimétrica e morfológica de órgãos internos,

189
refletindo secundariamente na genitália), a seleção sexual antagônica via coevolução (partindo
do pressuposto de divisão de funções entre os lados direito e esquerdo) e da seleção sexual por
meio da alteração de posições de acasalamento (a assimetria morfológica como uma
compensação mecânica para mudanças evolutivas e comportamentais da posição de
acasalamento) (Huber et al., 2007; Huber, 2010). Cada uma se suporta em casos específicos,
porém a última parece ser a mais recorrente explicação para o surgimento da assimetria na
genitália de insetos (Huber, 2010).
Quanto às fêmeas, a assimetria nas estruturas genitais é muito pouco estudada.
Considera-se que seja um estado raro, menos infrequente apenas em Phoridae, em que a
presença da vagina e escleritos vaginais assimétricos é provavelmente associada à assimetria
distifálica dos machos (Buck, 2001; Buck & Disney, 2001; Huber et al., 2007). Assim, estudos
futuros da morfologia externa e interna das fêmeas de Oxysarcodexia, cujos machos
apresentam assimetria podem vir a corroborar tal hipótese também para este gênero de
dípteros, uma vez que a modificação nos machos está associada diretamente ao falo. Da
mesma forma, uma análise da posição de cópula pode facilitar a compreensão de como e se as
estruturas assimétricas podem modular a interação entre machos e fêmeas de Oxysarcodexia.
Apesar da dificuldade de reconhecimento e desconhecimento de muitas espécies, o estudo
morfológico das fêmeas de Oxysarcodexia à luz da filogenia, assim como das larvas, poderá
contribuir significativamente para um melhor entendimento da relação entre as espécies.
AGRADECIMENTOS
À Dra. C.A. Mello-Patiu por possibilitar o exame dos espécimes depositados no Museu
Nacional/Universidade Federal do Rio de Janeiro; à MsC. E. Buenaventura por possibilitar o
exame dos espécimes depositados no Centro Tecnológico de Antioquia, Instituição
Universitária; e ao Dr. K.A. Johanson por possibilitar o exame dos espécimes depositados no
Museu de História Natural Sueco.
Esta pesquisa foi apoiada pela Fundação CAPES, Ministério da Educação do Brasil
(processo #0906/12-3).

190
REFERÊNCIAS BIBLIOGRÁFICAS
Blackith, R.; Blackith, R.; Pape, T. 1997. Taxonomy and systematics of Helicophagella Enderlein,
1928 (Diptera: Sarcophagidae) with the description of a new species and a revised catalogue.
Studia Dipterologica 4: 383–434.
Buck, M. & Disney, R.H.L. 2001. Revision of the Megaselia giraudii and M. densior species
complexes of Europe, including ecological notes (Diptera, Phoridae). Beiträge zur Entomologie,
51: 73–154.
Buck, M. 2001. Identification of females of European Conicera Meigen, with a discussion of certain
features of the reproductive anatomy of female Phoridae (Diptera). Deutsche Entomologische
Zeitschrift, 48: 69–81.
Cheung, D.K.-B., Brunke, A.J., Akkari, N., Souza, C.M., Pape, T. 2013. Rotational scanning electron
micrographs (rSEM): A novel and accessible tool to visualize and communicate complex
morphology. ZooKeys, 328: 47–57.
Chvála, M. 1975. The Tachydromiinae (Dipt. Empididae) of Fennoscandia and Denmark. Fauna
Entomologica Scandinavica, 3: 1–336.
Couri, M.S., Pont, A.C. 2000. Cladistic analysis of Coenosiini (Diptera: Muscidae: Coenosiinae).
Systematic Entomology, 25: 373–392.
Cumming, J.M. & Cooper, B.E. 1992. A revision of the Nearctic species of the Tachydromiinae fly
genus Stilpon Loew (Diptera: Empidoidea). The Canadian Entomologist, 124: 951–998.
Dodge, H.R. 1965. The Sarcophagidae (Diptera) of the West Indies. II. Jamaica. Annals of the
Entomological Society of America, 58: 497–517.
Dodge, H.R. 1966. Some new or little-known Neotropical Sarcophagidae (Diptera), with a review of
the genus Oxysarcodexia. Annals of the Entomological Society of America, 59: 674–701.
Downes, W.L. 1955. Notes on the morphology and classification of the Sarcophagidae and other
calyptrates (Diptera). Iowa Academy of Science, 62 (5): 514–538.
Giroux, M. & Wheeler, T.A. 2009. Systematics and phylogeny of the subgenus Sarcophaga
(Neobellieria) (Diptera: Sarcophagidae). Annals of the Entomological Society of America, 102 (4):
567–587.
Giroux, M., Pape, T. & Wheeler, T.A. 2010. Towards a phylogeny of the flesh flies (Diptera:
Sarcophagidae): morphology and phylogenetic implications of the acrophallus in the subfamily
Sarcophaginae. Zoological Journal of the Linnean Society, 158: 740–778.
Goloboff, P.A.; Farris, J.S.; Nixon, K.C. 2008. TNT, a free program for phylogenetic analysis.
Cladistics, 24: 774–786.

191
Huber, B.A. 2010. Mating positions and the evolution of asymmetric insect genitalia. Genetica, 138:
19–25.
Huber, B.A.; Sinclair, B.J.; Schmitt, M. 2007. The evolution of asymmetric genitalia in spiders and
insects. Biological Reviews, 82: 647–698.
Kutty, S.N.; Pape, T.; Pont, A.; Wiegmann, B.M.; Meier, R. 2008. The Muscoidea (Diptera:
Calyptratae) are paraphyletic: evidence from four mitochondrial and four nuclear genes. Molecular
Phylogenetics and Evolution, 49: 639–652.
Kutty, S.N.; Pape, T.; Pont, A.; Wiegmann, B.M.; Meier, R. 2010. Molecular phylogeny of the
Calyptratae (Diptera: Cyclorrhapha) with an emphasis on the superfamily Oestroidea and the
position of Mystacinobiidae and McAlpine‟s fly. Systematic Entomology, 35: 614–635.
Lopes, H.S. 1946. Contribuição ao conhecimento das espécies do gênero Oxysarcodexia Towsend,
1917 (Diptera Sarcophagidae). Boletim Escolar Nacional de Vetores, 1: 62–134.
Lopes, H.S. 1973. Notes on some Neotropical Sarcophagidae studied by H. R. Dodge (Diptera). Anais
da Academia Brasileira de Ciências, 45: 293–299.
Lopes, H.S. 1975a. New or little known Oxysarcodexia (Diptera, Sarcophagidae). Revista Brasileira de
Entomologia, 35 (3), 461–483.
Lopes, H.S. 1975b. On female holotypes of some American species described by Francis Walker and J.
Macquart (Diptera, Sarcophagidae, Calliphoridae). Revista Brasileira de Biologia, 34 (2): 535–550.
Lopes, H.S. 1976. On the holotypes, mostly females, of some Sarcophagidae (Diptera) described by
Francis Walker. Revista Brasileira de Biologia, 36: 629–641.
Lopes, H.S. 1978. On the Types of Some Neotropical Sarcophagidae described by H. R. Dodge
(Diptera). Revista Brasileira de Biologia, 38 (2): 501–507.
Lopes, H.S. 1982. The importance of the mandible and clypeal arch of the first instar larvae in the
classification of the Sarcophagidae (Diptera). Revista Brasileira de Entomologia, 26: 293–326.
Maddison, W.P. & Maddison, D.R. 2011. Mesquite: A modular system for evolutionary analysis.
Version 2.75. http://mesquiteproject.org.
McAlpine, J.F. 1981. Morphology and terminology. In: McAlpine, J.F., Peterson, B.V., Shewell, G.E.,
Teskey, H.J., Vockeroth, J.R. & Wood, D.M. (eds.), Manual of Nearctic Diptera. Vol. 1.
Monograph 27, Agriculture Canada, pp. 9–63.
Mello-Patiu, C.A. & Pape, T. 2000. Definitions of Dexosarcophaga Townsend 1917 and
Sarcofahrtiopsis Hall 1933, including two new species and a redescription of Sarcofahrtiopsis
cuneata (Townsend 1935) (Diptera: Sarcophagidae). Boletín de Entomología Venezolana, 15 (2):
181–194.

192
Metcalf, C. L. 1921. The genitalia of male Syrphidae: their morphology, with special reference to its
taxonomic significance. Annals of the Entomological Society of America, 14: 169–225.
Nakayama, H. 2008. Systematic and morphological evaluations of a new species of Kuenburgia
Schmitz (Diptera: Phoridae) with remarkable asymmetry of the male genitalia, including a
bifurcated left surstylus. Entomological Science, 11: 179–188.
Nakayama, H. 2012. Complex asymmetric male genitalia of Anevrina Lioy (Diptera: Phoridae).
Arthropod Structure & Development, 41: 35–49.
O‟Hara, J.E. 2002. Revision of the Polideini (Tachinidae) of America north of Mexico. Studia
dipterologica, Supplement 10: 1–170.
Palmer, A.R. 2004. Symmetry breaking and the evolution of development. Science, 306: 828–833.
Palmer, A.R. 2009. Animal asymmetry. Current Biology, 19 (12): 473–477.
Pape, T. 1992. Phylogeny of the Tachinidae family-group (Diptera: Calyptratae). Tijdschrift voor
Entomologie, 135: 43–86.
Pape, T. 1996. Catalogue of the Sarcophagidae of the world (Insecta: Diptera). Memoirs on
Entomology, International, 8, 1–558.
Rafael, J.A. & De Meyer, M. 1992. Generic classification of the family Pipunculidae (Diptera): a
cladistic analysis. Journal of Natural History, 26: 637–658.
Roback, S.S. 1954. The evolution and taxonomy of the Sarcophaginae (Diptera, Sarcophagidae).
Illinois Biological Monographs, 23: 1–181.
Soares, W.F. & Mello-Patiu, C.A. 2010. Two new Neotropical species of the genus Oxysarcodexia
Towsend (Diptera, Sarcophagidae). Revista Brasileira de Entomologia, 54 (1): 72–75.
Souza, C.M.; Pape, T. & Thyssen, P.J. In prep. On Oxysarcodexia (Diptera: Sarcophagidae): a
taxonomic conspectus with the description of six new species.
Stamper, T., Dahlem, G.A., Cookman, C., & Debry R.W. 2012. Phylogenetic relationships of flesh
flies in the subfamily Sarcophaginae based on three mtDNA fragments (Diptera: Sarcophagidae).
Systematic Entomology, 38 (1): 35–44.
Tibana, R. & Mello, C.A. 1983. Estudo sobre as fêmeas de Oxysarcodexia do grupo Peltata (Diptera,
Sarcophagidae). Revista Brasileira de Biologia, 43 (3), 241–250.
Triplehorn, C.A. & Johnson, N.F. 2005. Collecting, preserving, and studying insects. In: Triplehorn,
C.A. & Johnson, N.F. 2005. Borror and DeLong's introduction to the study of insects. 7th ed.
Thomson Brooks/Cole, California, USA, pp. 745–778.

193
Wells, J.D.; Pape, T.; Sperling, F. 2001. DNA-based identification and molecular systematics of
forensically important Sarcophagidae (Diptera). Journal of Forensic Sciences, 46 (5): 1098–1102.
Whitmore, D., Pape, T. & Cerretti, P. 2013. Phylogeny of Heteronychia: the largest lineage of
Sarcophaga (Diptera: Sarcophagidae). Zoological Journal of the Linnean Society, 169: 604–639.

194
Figura 1 – Árvore de consenso resultante das 12 árvores mais parcimoniosas recuperadas da
análise da matriz de caracteres completa para espécies de Oxysarcodexia Townsend, 1917
(Diptera: Sarcophagidae), por meio do programa TNT, considerando pesos iguais para os
caracteres (análise “traditional search” com RAM 1.000 Mb; memória de 99.999 árvores;
“random seed” de 50.000 árvores, 1.000 réplicas; e 1.000 árvores salvas por replicação).

195
Figura 2 – Árvore mais parcimoniosa recuperada da análise da matriz de caracteres completa
para espécies de Oxysarcodexia Townsend, 1917 (Diptera: Sarcophagidae), por meio do
programa TNT, considerando pesos iguais para os caracteres (análise “new technology search”
com RAM 1.000 Mb; memória de 99.999 árvores; “ratched search” com 1.000 sequências
adicionadas inicialmente e 1.000 árvores salvas por replicação).

196
Figura 3 – Árvore de consenso resultante das 12 árvores mais parcimoniosas recuperada da
análise da matriz de caracteres completa para espécies de Oxysarcodexia Townsend, 1917
(Diptera: Sarcophagidae), por meio do programa TNT, considerando pesos iguais para os
caracteres e suporte Bremer calculado para 50.000 árvores sub-ótimas com até 10 passos a
mais que a árvore mais curta obtida (análise “traditional search” com RAM 1.000 Mb;
memória de 99.999 árvores; “random seed” de 50.000 árvores, 1.000 réplicas; e 1.000 árvores
salvas por replicação).

197
Figura 4 – Árvore de consenso resultante das 3 árvores mais parcimoniosas recuperadas da
análise da matriz de caracteres completa para espécies de Oxysarcodexia Townsend, 1917
(Diptera: Sarcophagidae), por meio do programa TNT, considerando pesos iguais para os
caracteres e suporte Bremer calculado para 50.000 árvores sub-ótimas com até 10 passos a
mais que a árvore mais curta obtida (análise “new technology search” com RAM 1.000 Mb;
memória de 99.999 árvores; “random seed” de 50.000 árvores, 1.000 réplicas; e 1.000 árvores
salvas por replicação).

198
Figura 5 – Árvore mais parcimoniosa recuperada da análise da matriz de caracteres completa
para espécies de Oxysarcodexia Townsend, 1917 (Diptera: Sarcophagidae), por meio do
programa TNT, considerando pesos diferenciados para os caracteres (k=3) (análise “traditional
search” com “implied weight” (k=3), RAM 1.000 Mb; memória de 99.999 árvores; “ratched
search” com 1.000 sequências adicionadas inicialmente e 1.000 árvores salvas por replicação).

199
Figura 6 – Árvore mais parcimoniosa recuperada da análise da matriz de caracteres completa
para espécies de Oxysarcodexia Townsend, 1917 (Diptera: Sarcophagidae), por meio do
programa TNT, considerando pesos diferenciados para os caracteres (k=3) (análise “new
technology search” com “implied weight” (k=3), RAM 1.000 Mb; memória de 99.999 árvores;
“ratched search” com 1.000 sequências adicionadas inicialmente e 1.000 árvores salvas por
replicação).

200
Figura 7 – Árvore de consenso resultante das 14 árvores mais parcimoniosas recuperadas da
análise da matriz de caracteres apenas da terminália, para espécies de Oxysarcodexia
Townsend, 1917 (Diptera: Sarcophagidae), por meio do programa TNT, considerando pesos
iguais para os caracteres e suporte Bremer calculado para 50.000 árvores sub-ótimas com até
10 passos a mais que a árvore mais curta obtida (análise “traditional search” com RAM 1.000
Mb; memória de 99.999 árvores; “ratched search” com 1.000 sequências adicionadas
inicialmente e 1.000 árvores salvas por replicação).

201
Figura 8 – Árvore mais parcimoniosas recuperadas da análise da matriz de caracteres apenas
da terminália, para espécies de Oxysarcodexia Townsend, 1917 (Diptera: Sarcophagidae), por
meio do programa TNT, considerando pesos implicados (k=3) para os caracteres e suporte
Bremer calculado para 50.000 árvores sub-ótimas com até 10 passos a mais que a árvore mais
curta obtida (análise “traditional search” com RAM 1.000 Mb; memória de 99.999 árvores;
“ratched search” com 1.000 sequências adicionadas inicialmente e 1.000 árvores salvas por
replicação).

202
Figura 9 – Terminália assimétrica dos machos de Oxysarcodexia Townsend, 1917. A)
Oxysarcodexia corolla Dodge, 1965 (reproduzido a partir de Lopes, 1978); B) Oxysarcodexia
edwardsi Lopes, 1946; C) Oxysarcodexia fringidea (Curran & Walley, 1934); D)
Oxysarcodexia ramosa (Reinhard, 1939) (reproduzido a partir de Dodge, 1966); E)
Oxysarcodexia sarcinata Lopes, 1953; F) Oxysarcodexia timida (Aldrich, 1916); G)
Oxysarcodexia varia (Walker, 1836); H) Oxysarcodexia villosa Lopes, 1946.
A
C
B
|
D
E
F
G
H

203
Figura 10 – Falos assimétricos submetidos à técnica de microscopia eletrônica de varredura.
A) Oxysarcodexia fringidea (Curran & Walley, 1934) (7,0kV; x65; 41,7mm); B)
Oxysarcodexia timida (Aldrich, 1916) (7,0kV; x65; 41,2mm); C) Oxysarcodexia varia
(Walker, 1836) (7,0kV; x65; 39,8mm). Ver também <http://dkbdigitaldesigns.com/
semrotate/sem.html>.
A B
C

204
APÊNDICE
APÊNDICE I. Espécies do gênero Oxysarcodexia Townsend, 1917, Dexosarcophaga Townsend,
1917 e Ravinia Robineau-Desvoidy, 1863 (Diptera: Sarcophagidae) examinadas para o
desenvolvimento da matriz de caracteres morfológicos, com indicação do local de origem
(estado ou província e país) e depositário dos espécimes examinados entre parênteses.
Onde: CE-TdeA = Tecnológico de Antioquia, Institución Universitaria, Medellín, Colômbia; L2B-DBA =
Laboratório de Entomologia do Departamento de Biologia Animal, Universidade Estadual de Campinas,
Campinas, São Paulo, Brasil; MNRJ = Museu Nacional/Universidade Federal do Rio de Janeiro, Rio de Janeiro,
Brasil; NMR = Swedish Museum of Natural History, Estocolmo, Suécia; ZMUC = Zoological Museum of
Copenhagen, Copenhagen, Dinamarca.
Espécie Local de origem e depositário
Dexosarcophaga transita (Townsend, 1917)
Mogi Guaçu, SP, Brasil (L2B-DBA)
Ipameri, GO, Brasil (L2B-DBA)
Uberlândia, MG, Brasil (L2B-DBA)
Oxysarcodexia admixta (Lopes, 1933) Jundiaí, SP, Brasil (L2B-DBA)
Oxysarcodexia adunca Lopes, 1975 Linhares, ES, Brasil (NRM)
Província de Napo, Equador (NRM)
Oxysarcodexia afficta (Wulp, 1895) Otavalo, Equador (NRM)
Chiapa de Corzo, México (ZMUC)
Oxysarcodexia amorosa (Schiner, 1868) Angra dos Reis, RJ, Brasil (NMR)
Angra dos Reis, RJ, Brasil (MNRJ)
Oxysarcodexia angrensis (Lopes, 1933) Mogi Guaçu, SP, Brasil (L2B-DBA)
Campinas, SP, Brasil (L2B-DBA)
Oxysarcodexia augusta Lopes, 1946 Belo Horizonte, MG, Brasil (NRM)
Oxysarcodexia aura (Hall, 1937)
Campinas, GO, Brasil (NRM)
Pirapora, MG, Brasil (MNRJ)
Uberlândia, MG, Brasil (L2B-DBA)
Oxysarcodexia aurata (Macquart, 1851) Província Nord, Nova Caledônia (NRM)
Society Is., Tahiti (ZMUC)
Oxysarcodexia avuncula (Lopes, 1933) Campinas, SP, Brasil (L2B-DBA)
Extrema, MG, Brasil (L2B-DBA)
Oxysarcodexia bakeri (Aldrich, 1916) Antioquia, Colômbia (CE-TdeA)
Cuba (MNRJ)

205
Continuação Apêndice I.
APÊNDICE I. Espécies do gênero Oxysarcodexia Townsend, 1917, Dexosarcophaga Townsend,
1917 e Ravinia Robineau-Desvoidy, 1863 (Diptera: Sarcophagidae) examinadas para o
desenvolvimento da matriz de caracteres morfológicos, com indicação do local de origem (estado
ou província e país) e depositário dos espécimes examinados entre parênteses.
Onde: CE-TdeA = Tecnológico de Antioquia, Institución Universitaria, Medellín, Colômbia; L2B-DBA =
Laboratório de Entomologia do Departamento de Biologia Animal, Universidade Estadual de Campinas,
Campinas, São Paulo, Brasil; MNRJ = Museu Nacional/Universidade Federal do Rio de Janeiro, Rio de
Janeiro, Brasil; NMR = Swedish Museum of Natural History, Estocolmo, Suécia; ZMUC = Zoological Museum
of Copenhagen, Copenhagen, Dinamarca.
Espécie Local de origem e depositário
Oxysarcodexia berlai Lopes, 1975 Avispas, Madre de Dios, Peru (NRM)
Igarapé, Paraqueú Rosário, MA, Brasil (MNRJ)
Oxysarcodexia bicolor Lopes, 1946 Petrópolis, RJ, Brasil (MNRJ; NMR)
Campos do Jordão, SP, Brasil (MNRJ)
Oxysarcodexia carvalhoi Lopes, 1946 Campinas, SP, Brasil (L2B-DBA)
Mogi Guaçu, SP, Brasil (L2B-DBA)
Oxysarcodexia chaetopygialis (Williston, 1896) Majorca, St. Vicent (MNRJ; ZMUC)
Oxysarcodexia cingarus (Aldrich, 1916) Great Falls, USA (ZMUC)
La Fayette, La., USA (MNRJ)
Oxysarcodexia conclausa (Walker, 1861) Hidalgo Co., Texas, USA (ZMUC)
Palmira, Colombia (MNRJ)
Oxysarcodexia confusa Lopes, 1946
Petrópolis, RJ, Brasil (NRM; ZMUC)
Iguassú, PR, Brasil (NRM)
São Paulo, SP, Brasil (MNRJ)
Oxysarcodexia culmiforceps Dodge, 1966 Extrema, MG, Brasil (L2B-DBA)
Oxysarcodexia culminata (Aldrich, 1916) Cerro de Punta, Porto Rico (NRM)
Oxysarcodexia diana (Lopes, 1933)
Antioquia, Colômbia (CE-TdeA)
Belo Horizonte, MG, Brasil (NRM)
Grajaú, RJ, Brasil (MNRJ)
Oxysarcodexia fluminensis Lopes, 1946 Antioquia, Colômbia (CE-TdeA)
Oxysarcodexia fringidea (Curran & Walley, 1934) Amazonas, Colômbia (NMR)
Oxysarcodexia galeata (Aldrich, 1916) Madson Co., Alabama, USA (NMR)

206
Continuação Apêndice I.
APÊNDICE I. Espécies do gênero Oxysarcodexia Townsend, 1917, Dexosarcophaga Townsend,
1917 e Ravinia Robineau-Desvoidy, 1863 (Diptera: Sarcophagidae) examinadas para o
desenvolvimento da matriz de caracteres morfológicos, com indicação do local de origem (estado
ou província e país) e depositário dos espécimes examinados entre parênteses.
Onde: CE-TdeA = Tecnológico de Antioquia, Institución Universitaria, Medellín, Colômbia; L2B-DBA =
Laboratório de Entomologia do Departamento de Biologia Animal, Universidade Estadual de Campinas,
Campinas, São Paulo, Brasil; MNRJ = Museu Nacional/Universidade Federal do Rio de Janeiro, Rio de
Janeiro, Brasil; NMR = Swedish Museum of Natural History, Estocolmo, Suécia; ZMUC = Zoological Museum
of Copenhagen, Copenhagen, Dinamarca.
Espécie Local de origem e depositário
Oxysarcodexia grandis Lopes, 1946 Antioquia, Colômbia (CE-TdeA)
Oxysarcodexia injuncta (Walker, 1857)
Extrema, MG, Brasil (L2B-DBA)
Jundiaí, SP, Brasil (L2B-DBA)
Rio Japura, AM, Brasil (NRM)
Oxysarcodexia insolita Lopes, 1946 Província de Napo, Equador (NRM)
Oxysarcodexia intona (Curran & Walley, 1934) Rio de Janeiro, RJ, Brasil (NRM)
Guarapari, ES, Brasil (ZMUC)
Oxysarcodexia major Lopes, 1946 Antioquia, Colômbia (CE-TdeA)
Oxysarcodexia mitifica Lopes, 1953 Antioquia, Colômbia (CE-TdeA)
Oxysarcodexia modesta Lopes, 1946 Rio de Janeiro, RJ, Brasil (MNRJ; NRM)
Mazagão, AP, Brasil (MNRJ)
Oxysarcodexia nitida Soares & Mello-Patiu, 2010 Província de Napo, Equador (NRM)
Oxysarcodexia notata Soares & Mello-Patiu, 2010 Província de Napo, Equador (NRM)
Oxysarcodexia occulta Lopes, 1946 Mogi Guaçu, SP, Brasil (L2B-DBA)
Oxysarcodexia parva Lopes, 1946 Campinas, SP, Brasil (L2B-DBA)
Oxysarcodexia paulistanensis (Mattos, 1919) Campinas, SP, Brasil (L2B-DBA)
Oxysarcodexia peltata (Aldrich, 1916) Cuba (ZMUC)
Oxysarcodexia perneta (Walker, 1861)
San Cristobal, Chiapas, México (ZMUC)
México D.F., México (MNRJ)
Cuernavaca, Morelos, México (MNRJ)
Oxysarcodexia plebeja Lopes, 1946 Antioquia, Colômbia (CE-TdeA)
San Cristobal, Chiapas, México (MNRJ)

207
Continuação Apêndice I.
APÊNDICE I. Espécies do gênero Oxysarcodexia Townsend, 1917, Dexosarcophaga Townsend,
1917 e Ravinia Robineau-Desvoidy, 1863 (Diptera: Sarcophagidae) examinadas para o
desenvolvimento da matriz de caracteres morfológicos, com indicação do local de origem (estado
ou província e país) e depositário dos espécimes examinados entre parênteses.
Onde: CE-TdeA = Tecnológico de Antioquia, Institución Universitaria, Medellín, Colômbia; L2B-DBA =
Laboratório de Entomologia do Departamento de Biologia Animal, Universidade Estadual de Campinas,
Campinas, São Paulo, Brasil; MNRJ = Museu Nacional/Universidade Federal do Rio de Janeiro, Rio de
Janeiro, Brasil; NMR = Swedish Museum of Natural History, Estocolmo, Suécia; ZMUC = Zoological Museum
of Copenhagen, Copenhagen, Dinamarca.
Espécie Local de origem e depositário
Oxysarcodexia riograndensis Lopes, 1946 Campinas, SP, Brasil (L2B-DBA)
Oxysarcodexia sarcinata Lopes, 1953
Calovébora, Província de Veraguas, Panamá (NRM)
Caldas, Antioquia, Colômbia (CE-TdeA)
Cerro del Aguacate, Província de Alajuela,
Costa Rica (ZMUC)
Turialba, Cartago, Costa Rica (MNRJ)
Oxysarcodexia similata Lopes & Tibana, 1987
Punta Charme, Província do Panamá, Panamá
(NRM)
Turialba, Cartago, Costa Rica (MNRJ)
Oxysarcodexia terminalis (Wiedemann, 1830) Brasília, DF, Brasil (NMR)
Petrópolis, RJ, Brasil (MNRJ)
Oxysarcodexia thornax (Walker, 1849) Campinas, SP, Brasil (DBA-L2B)
Oxysarcodexia timida (Aldrich, 1916) Aragua, Venezuela (ZMUC)
Oxysarcodexia trivialis (Wulp, 1895) Puntaneras, Costa Rica (ZMUC)
San José, Costa Rica (ZMUC)
Oxysarcodexia varia (Walker, 1836) Chubut, Argentina (NRM)
Oxysarcodexia ventricosa (Wulp, 1895) Alabama, USA (NRM)
Flórida, USA (ZMUC)
Oxysarcodexia vittata (Walker, 1836) Jundiaí, SP, Brasil (L2B-DBA)
Nova Teutonia, MG, Brasil (ZMUC)
Oxysarcodexia xanthosoma (Aldrich, 1916) Província de Napo, Equador (NRM)
Campinas, SP, Brasil (L2B-DBA)

208
Continuação Apêndice I.
APÊNDICE I. Espécies do gênero Oxysarcodexia Townsend, 1917, Dexosarcophaga Townsend,
1917 e Ravinia Robineau-Desvoidy, 1863 (Diptera: Sarcophagidae) examinadas para o
desenvolvimento da matriz de caracteres morfológicos, com indicação do local de origem (estado
ou província e país) e depositário dos espécimes examinados entre parênteses.
Onde: CE-TdeA = Tecnológico de Antioquia, Institución Universitaria, Medellín, Colômbia; L2B-DBA =
Laboratório de Entomologia do Departamento de Biologia Animal, Universidade Estadual de Campinas,
Campinas, São Paulo, Brasil; MNRJ = Museu Nacional/Universidade Federal do Rio de Janeiro, Rio de Janeiro,
Brasil; NMR = Swedish Museum of Natural History, Estocolmo, Suécia; ZMUC = Zoological Museum of
Copenhagen, Copenhagen, Dinamarca.
Espécie Local de origem e depositário
Oxysarcodexia zayasi Dodge, 1956 Santiago, Cuba (NRM)
Las Villas Trinidad, Cuba (MNRJ)
Oxysarcodexia n. sp. 1 Amazonas, Colômbia (ZMUC)
Província de Napo, Equador (ZMUC)
Oxysarcodexia n. sp. 2 Montsinery, Guiana Francesa (ZMUC)
Oxysarcodexia n. sp. 3 Puntaneras, Monteverde, Costa Rica (ZMUC)
Oxysarcodexia n. sp. 6 Província de Napo, Equador (ZMUC)
Ravinia belforti (Prado & Fonseca, 1932) Campinas, SP, Brasil (L2B-DBA)

209
APÊNDICE II. Lista de caracteres morfológicos baseada no exame de machos de Oxysarcodexia
Townsend, 1917 (Diptera: Sarcophagidae).
1. Cabeça, área postocular, polinosidade: (0) prateada; (1) dourada; (2) dourada
esbranquiçada.
2. Cabeça, cerda ocelar: (0) bem desenvolvida (= maior ou mesmo tamanho que as menores
cerdas frontais); (1) não bem desenvolvidas (= menor que as menores cerdas frontais).
3. Tórax, cerda apical escutelar: (0) presente; (1) ausente.
4. Tórax, asa, setosidade na veia R4+5: (0) presente na ½ proximal; (1) presente nos ⅔
proximais; (2) presente nos ¾ proximais.
5. Tórax, cerdas pós-suturais dorsocentrais: (0) 2 bem diferenciadas+1–2–3 pequenas; (1) 3
bem diferenciadas (uma pequena cerda pode estar presente).
6. Tórax, pernas, cor: (0) enegrecidas; (1) marrons; (2) amareladas.
7. Tórax, pernas, fêmur médio, número de espinhos no ctenídio: (0) 4; (1) 5; (2) 6; (3) 7; (4)
9.
8. Tórax, pernas, fêmur posterior, cerdas anteroventrais: (0) presentes somente na metade
apical; (1) presentes em toda a extensão.
9. Abdômen, tergito 3, número de cerdas laterais marginais: (0) 1; (1) 2; (2) 3.
10. Abdômen, tergito 4, número de cerdas laterais marginais: (0) 1; (1) 2; (2) 3.
11. Abdômen, tergito 4, número de cerdas medianas marginais: (0) 0; (1) 1; (2) 2.
12. Abdômen, tergito 5, polinosidade: (0) completamente dourada; (1) parcialmente dourada;
(2) não dourada.
13. Abdômen, esternitos 2–4, formato: (0) retangular; (1) quadrado.
14. Abdômen, esternitos 2–4, membrana entre os esternitos: (0) visível; (1) não visível.
15. Abdômen, esternito 5, cor: (0) completamente amarelo; (1) parcialmente amarelo; (2) não
amarelo.
16. Abdômen, esternito 5, bordas da fenda: (0) paralelas; (1) divergentes.
17. Abdômen, esternito 5, ornamentação nos ramos: (0) setosidade; (1) cerdas; (2) setosidade
+ cerdas.
18. Abdômen, esternito 5, posição das cerdas: (0) no ápice do ramo; (1) na metade apical dos
ramos; (2) ao longo da borda dos ramos; (3) espalhados pela superfície dos ramos.

210
19. Abdômen, esternito 5, tamanho dos ornamentos (cerdas ou sétulas): (0) micro (menor que
ou cerca de 0,1 x o comprimento da menor sétula na superfície do tergito 4); (1) pequeno
(cerca de 0,2–0,5 x o comprimento da menor sétula na superfície do tergito 4); (2) grande
(praticamente o mesmo comprimento da menor sétula na superfície do tergito 4).
20. Abdômen, esternito 5, espaço entre os ramos: (0) uniforme; (1) não uniforme.
21. Abdômen, esternito 5, espaço entre os ramos: (0) convergindo para o ápice; (1) não
convergindo para o ápice.
22. Abdômen, esternito 5, espaço entre os ramos: (0) alargado na base; (1) não alargado na
base.
23. Abdômen, esternito 5, comprimento dos ramos: (0) curtos; (1) longos.
24. Abdômen, esternito 5, largura dos ramos: (0) estreitos; (1) medianos; (2) largos.
25. Abdômen, esternito 5, invaginação da margem distal (visível lateralmente aos ramos): (0)
presente; (1) ausente.
26. Abdômen, esternito 5, “janela” (abertura visível entre as bordas internas dos ramos): (0)
estreita; (1) intermediária; (2) larga.
27. Abdômen, sintergosternito 7+8, cor: (0) marrom amarelado; (1) marrom enegrecido.
28. Abdômen, sintergosternito 7+8, formato dorsal: (0) linear; (1) sinuoso.
29. Abdômen, sintergosternito 7+8, cerdas na margem distal: (0) 6; (1) 7; (2) 8; (3) 10; (4) 4;
(5) 9.
30. Terminália, surstilo, formato: (0) triangular, (1) retangular.
31. Terminália, surstilo, cerda discal: (0) presente; (1) ausente.
32. Terminália, cerco, formato (vista lateral): (0) curvado para trás; (1) sinuoso; (2) reto.
33. Terminália, cerco, ápice (vista lateral): (0) pontiagudo; (1) expandido; (2) normal (mesmo
tamanho que a área mediana).
34. Terminália, cerco, expansão apical (vista lateral): (0) reto; (1) oblíquo para cima; (2)
côncavo.
35. Terminália, cerco, cerdas na face ventral (vista lateral): (0) presente na metade apical; (1)
presente na metade basal; (2) presente em toda a extensão; (3) ausente somente na porção
mediana; (4) presente no ⅓ apical.

211
36. Terminália, cerco, tamanho das cerdas dorsais (vista lateral): (0) mesmo tamanho em toda
a extensão; (1) mais longas na metade basal; (2) mais longas no ⅓ basal; (3) mais longas nos
⅔ basais.
37. Terminália, cerco, formato basal (vista posterior): (0) arredondado; (1) não arredondado.
38. Terminália, cerco, formato apical (vista posterior): (0) mesmo tamanho que a porção
mediana; (1) menor que a porção mediana; (2) maior que a porção mediana.
39. Terminália, cerco, conformação (vista posterior): (0) paralela; (1) divergente.
40. Terminália, cerco, espaço entre os cercos no nível apical (vista posterior): (0) pequeno; (1)
médio; (2) grande; (3) sem espaço entre os cercos.
41. Terminália, cerco, tufos de sétulas lateromedialmente no ápice (vista posterior): (0)
presente; (1) ausente.
42. Terminália, cerco, notável constrição na porção mediana (vista posterior): (0) presente; (1)
ausente.
43. Terminália, pênis; tamanho da expansão triangular (“tooth-like”) localizada acima da
vésica (vista lateral): (0) pequena (não ultrapassa a margem distal do tubo fálico); (1) grande
(ultrapassa a margem distal do tubo fálico).
44. Terminália, pênis/distifalo, concavidade ventroapical (vista lateral): (0) presente; (1)
ausente.
45. Terminália, pênis/distifalo, borda ventroapical (vista lateral): (0) serrada; (1) reta.
46. Terminália, pênis/distifalo, sulco lateroapical (vista lateral): (0) presente; (1) ausente;
47. Terminália, pênis/distifalo, formato apical (vista lateral): (0) cônico; (1) arredondado; (2)
quadrado/retangular.
48. Terminália, pênis/distifalo, formato da margem dorsal (vista lateral): (0) reta; (1) sinuosa.
49. Terminália, pênis/distifalo, projeções ventroapicais (vista lateral): (0) presente; (1)
ausente.
50. Terminália, pênis/distifalo, dilatação dorsoapical (vista lateral): (0) presente; (1) ausente.
51. Terminália, pênis/distifalo, tamanho da dilatação dorsoapical (vista lateral): (0) pequena;
(1) média; (2) grande.
52. Terminália, pênis/distifalo, lobos laterais (vista lateral): (0) presente; (1) ausente.
53. Terminália, pênis/distifalo, expansões lateroapicais (vista dorsal): (0) presente; (1) ausente.
54. Terminália, pênis/distifalo, fenda apical (vista dorsal): (0) presente; (1) ausente.

212
55. Terminália, pênis/distifalo, tamanho da fenda apical (vista dorsal): (0) pequena; (1)
grande.
56. Terminália, pênis, sulco dorsal (vista dorsal): (0) presente; (1) ausente.
57. Terminália, pênis, comprimento do sulco dorsal (vista dorsal): (0) ao longo de toda a
extensão; (1) restrita aos ⅔ basais.
58. Terminália, pregonito, formato: (0) base expandida estreitando repentinamente no ápice;
(1) base expandida estreitando suavemente até o ápice; (2) base e ápice com praticamente a
mesma largura.
59. Terminália, pregonito, cor: (0) mesma cor em toda a extensão da estrutura; (1) ápice mais
escuro do que a base.
60. Terminália, pós-gonito; formato: (0) base expandida estreitando repentinamente no ápice;
(1) base expandida estreitando suavemente até o ápice.
61. Terminália, vésica, ramos: (0) simétricos; (1) assimétricos.
62. Terminália, vésica, lobos terminais: (0) bem desenvolvidos; (1) reduzidos.
63. Terminália, vésica, lobos laterais: (0) presentes; (1) ausentes.
64. Terminália, vésica, forma da margem ventral dos lobos laterais: (0) côncava; (1) convexa.
65. Terminália, vésica, formato dos lobos terminais: (0) filamentoso, ou pelo menos afilando
no ápice; (1) arredondada; (2) quadrada.
66. Terminália, vésica, textura dos lobos terminais: (0) membranosa; (1) parcialmente
membranosa e parcialmente esclerotizada; (2) esclerotizada.
67. Terminália, vésica, espinhos: (0) presentes; (1) ausentes.
68. Terminália, vésica, tamanho dos espinhos: (0) micro; (1) pequeno; (2) longo.
69. Terminália, vésica, posição dos espinhos: (0) presente nas faces dorsal e ventral; (1)
presente somente ao longo da borda dos lobos terminais; (2) presente somente na face ventral.
70. Terminália, vésica, projeção mediana do ramo principal: (0) presente; (1) ausente.
71. Terminália, vésica, formato do pico da projeção mediana do ramo principal: (0) angular,
(1) arredondada.
72. Terminália, vésica, comprimento da projeção mediana do ramo principal: (0) comprimento
horizontal igual ao vertical; (1) comprimento horizontal maior que o vertical; (2) comprimento
vertical maior que o horizontal.

213
73. Terminália, vésica, comprimento da margem apical do lobo terminal: (0) ultrapassa o
ponto de conexão com o ramo principal; (1) não ultrapassa o ponto de conexão com o ramo
principal.

214
APÊNDICE III. Matriz de dados provenientes dos caracteres morfológicos levantados a partir
do exame de machos de Oxysarcodexia Townsend, 1917 (Diptera: Sarcophagidae) ( _ = não se
aplica; ? = “missing data”).
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36
D. transita 1 0 0 1 0 0 2 1 0 1 0 1 0 0 2 1 0 - 1 1 1 1 0 2 0 2 0 1 2 0 0 2 0 - 0 3
R. belforti 1 1 1 1 0 1 4 1 2 2 1 1 0 0 2 1 2 0 0 1 1 1 1 2 1 2 0 0 0 1 1 1 0 - 0 3
O. admixta 1 0 0 0 0 0 1 1 0 1 1 1 1 0 2 0 0 - 0 0 1 1 1 2 1 1 0 1 1 0 0 2 1 1 2 2
O. adunca 2 1 1 1 0 0 2 1 0 0 1 0 0 0 0 0 2 0 1 1 1 0 1 1 0 0 0 0 2 0 0 1 1 1 3 1
O. afficta 2 1 1 1 0 0 2 0 1 1 2 1 1 1 0 0 0 - 1 1 0 1 1 0 0 1 0 1 2 0 0 1 1 1 2 2
O. amorosa 2 1 0 1 0 1 1 1 2 1 1 1 1 1 2 0 0 - 1 0 1 1 1 1 0 1 0 1 2 0 1 2 1 1 2 3
O. angrensis 1 0 1 1 0 0 2 1 2 1 1 0 1 0 1 0 2 0 0 0 1 1 1 1 0 0 0 0 2 0 1 2 0 1 3 3
O. augusta 1 1 0 1 1 0 0 1 1 1 1 2 0 1 1 0 2 0 0 0 1 1 1 1 0 1 0 0 0 0 0 2 1 1 2 3
O. aura 0 0 1 2 0 1 2 0 2 1 1 1 1 1 2 1 1 2 1 1 1 1 1 1 1 2 1 1 1 0 0 2 0 2 1 2
O. aurata 1 0 1 1 0 0 3 1 2 2 1 1 0 0 2 0 1 0 1 0 1 1 1 2 0 0 0 0 2 0 0 1 2 2 4 1
O. avuncula 1 0 0 2 0 0 0 1 2 2 1 1 1 0 1 0 2 0 0 1 1 0 1 1 0 1 0 0 2 0 0 1 0 2 4 3
O. bakeri 1 0 0 1 0 0 0 1 ? 2 ? 0 1 1 2 0 0 - 0 0 1 1 0 1 1 0 0 0 4 1 1 2 0 1 2 3
O. berlai 2 1 0 2 0 1 1 1 0 1 1 0 1 1 1 0 2 1 0 0 1 1 1 1 0 1 0 0 0 0 0 2 1 0 3 3
O. bicolor 1 1 1 1 0 1 2 0 0 1 1 2 0 0 2 0 0 - 0 1 0 1 0 0 0 0 0 0 5 0 0 1 2 1 3 1
O. carvalhoi 1 1 0 2 0 0 1 1 1 1 1 0 1 0 0 0 0 - 0 0 0 1 1 1 1 1 0 1 2 1 0 2 1 1 4 3
O. chaetopygialis 1 0 1 2 0 1 0 1 1 0 ? 2 1 1 0 0 2 2 2 0 1 1 0 1 1 1 0 1 0 0 0 1 0 1 4 1
O. cingarus 2 1 1 1 0 1 0 1 0 1 1 1 1 1 0 0 2 0 0 0 1 0 0 0 0 2 0 0 0 0 1 2 0 1 4 3
O. conclausa 2 1 1 1 0 1 1 1 2 1 1 0 0 0 0 0 0 - 0 0 0 1 1 1 1 1 0 0 3 0 0 2 1 1 4 3
O. confusa 1 0 1 1 0 1 1 1 1 2 1 2 1 1 0 0 0 - 1 0 0 1 0 1 1 1 0 1 2 0 0 2 1 1 3 0
O. culmiforceps 1 0 1 1 0 0 3 1 1 1 1 1 0 0 1 0 2 2 1 0 1 1 0 2 1 0 1 0 2 0 1 0 0 1 4 2
O. culminata ? 0 1 1 0 0 2 ? 1 2 1 2 1 1 1 0 0 - 1 0 1 1 0 2 1 2 0 0 1 0 0 0 0 1 4 1
O. diana 1 1 1 2 0 1 3 1 2 0 1 0 1 1 0 0 2 0 0 0 1 1 0 0 1 1 0 0 0 0 1 1 1 1 4 3
O. fluminensis 1 0 0 2 0 0 0 1 ? 0 ? 1 1 1 1 0 0 - 1 0 1 1 1 1 1 1 0 0 3 0 0 2 1 1 4 3
O. fringidea 2 1 1 1 0 0 3 1 2 2 1 0 1 0 0 0 1 0 1 1 1 0 1 2 1 0 0 0 1 0 0 2 1 1 4 1
O. galeata 0 0 1 1 0 1 1 1 0 0 0 0 0 1 0 1 2 0 0 1 1 1 0 1 1 2 0 0 1 0 0 1 0 1 4 2
O. grandis 1 0 0 2 1 0 1 1 ? 0 ? 0 1 1 2 0 0 - 1 1 1 0 1 0 0 1 0 0 2 0 0 2 0 1 4 3
O. injuncta 1 1 0 1 0 0 0 1 ? 1 ? 2 1 1 1 0 1 3 1 0 1 1 1 1 1 1 0 1 2 0 0 2 1 1 2 3
O. insolita 2 1 0 0 0 1 1 1 1 1 1 1 1 0 2 0 2 3 1 1 0 1 0 2 1 1 0 1 1 0 0 1 1 0 4 3
O. intona 1 1 1 2 0 1 3 1 ? 2 ? 1 0 1 2 0 1 0 1 0 1 1 1 2 1 0 0 0 1 0 0 1 1 2 4 1
O. major 1 1 0 0 0 0 2 1 1 1 1 1 1 1 0 0 2 3 1 0 1 1 1 2 1 2 0 0 1 1 0 1 2 1 3 1
O. mitifica 1 0 1 0 0 0 1 1 ? 1 ? 0 1 1 0 0 0 - 0 1 1 0 0 2 1 0 0 0 0 0 1 2 0 1 4 0
O. modesta 2 0 1 0 0 1 1 1 0 1 1 1 1 1 0 0 1 1 2 0 1 1 1 2 1 0 1 0 - 0 0 2 0 1 2 0
O. nitida 2 0 0 1 1 0 0 1 1 1 1 1 1 0 2 0 2 0 1 1 1 0 1 1 1 0 0 0 1 0 0 2 0 1 1 0
O. notata 1 0 0 1 1 1 0 1 0 1 1 0 1 0 0 0 2 3 2 0 1 1 1 1 1 1 0 0 4 0 0 2 0 1 3 3
O. occulta 1 1 1 2 0 0 1 1 0 1 1 1 1 0 0 0 0 - 1 1 0 1 1 1 1 0 0 0 2 0 1 0 2 1 4 3
O. parva 1 0 1 1 0 0 1 1 0 0 1 1 1 1 2 0 1 3 1 0 1 1 0 1 1 1 0 0 0 0 0 1 0 1 3 3
O. paulistanensis 1 1 0 1 0 0 1 1 1 1 1 2 1 0 1 0 0 - 1 0 1 1 1 1 1 2 0 1 5 0 0 1 1 1 2 3
O. peltata 1 0 1 1 0 1 1 0 0 0 0 1 0 1 1 0 1 0 1 0 1 1 1 2 0 0 0 0 0 0 0 0 2 2 4 2
O. perneta 1 1 0 0 0 1 1 1 0 1 1 0 1 1 1 0 2 0 1 0 1 1 0 1 1 0 0 0 2 0 0 2 0 1 2 1
O. plebeja 1 1 0 1 1 0 0 1 ? 1 ? 0 1 0 1 0 2 0 1 0 1 1 1 1 1 0 0 0 2 1 1 2 1 1 0 0
O. riograndensis 1 0 0 1 0 0 0 1 1 1 1 2 1 0 2 0 0 - 2 0 1 1 1 1 1 2 0 1 1 0 0 2 1 1 3 3
O. sarcinata 1 0 0 2 0 0 1 1 1 1 1 1 1 0 1 0 1 1 1 0 1 1 1 0 1 0 0 1 0 0 0 1 1 1 3 3
O. similata 2 0 0 2 0 0 1 1 1 0 1 1 1 1 2 0 0 - 0 1 1 0 0 1 1 1 0 0 2 0 0 2 1 1 2 1
O. terminalis 0 0 1 1 1 1 1 0 1 0 1 2 1 1 1 1 2 2 0 1 1 1 0 2 1 2 0 0 1 0 0 0 0 1 4 1
O. thornax 2 1 1 1 0 0 1 1 1 1 1 1 0 0 1 0 2 0 0 1 0 1 1 1 1 1 0 0 2 1 0 2 2 0 3 1
O. timida 1 1 1 2 0 0 2 1 1 0 1 1 0 1 0 0 2 0 1 0 1 1 1 1 1 0 0 1 3 0 0 2 1 1 2 2
O. trivialis 1 1 0 1 0 0 2 1 2 2 1 1 1 1 0 0 2 0 2 0 1 1 0 2 1 1 0 0 2 0 0 1 1 1 4 3
O. varia 1 0 1 0 1 0 2 1 0 1 0 1 0 1 2 1 2 2 2 1 1 1 0 2 1 2 1 1 2 1 0 1 1 2 2 3
O. ventricosa 0 1 1 2 0 2 1 1 2 1 1 1 1 1 0 0 2 0 0 0 1 1 0 0 1 1 0 1 2 0 0 2 1 1 4 2
O. vittata 1 0 0 2 1 0 1 ? 2 1 1 1 0 1 0 0 2 0 0 1 1 0 0 2 1 1 0 1 2 1 0 1 0 1 2 1
O. xanthosoma 2 1 0 1 0 0 1 1 2 1 1 1 1 1 1 0 0 - 1 1 1 0 1 1 1 1 0 0 1 0 0 2 1 1 3 3
O. zayasi 2 1 1 2 0 2 1 1 2 1 0 2 0 1 0 1 0 - 0 1 1 1 0 2 1 2 0 1 2 0 0 2 1 1 2 2
Oxysarcodexia n. sp. A 1 0 0 1 0 0 1 1 0 1 1 0 1 1 2 0 0 - 1 0 1 1 1 2 1 1 0 1 2 0 0 2 1 1 2 3
Oxysarcodexia n. sp. B 1 1 0 1 0 0 1 1 1 0 1 1 1 1 1 0 2 0 1 1 0 1 1 1 0 1 0 ? 5 0 0 2 1 1 2 0
Oxysarcodexia n. sp. C 1 1 1 0 0 0 2 1 1 2 0 2 0 1 2 1 2 2 1 1 1 1 0 2 0 2 1 1 3 0 0 1 0 1 1 0
Oxysarcodexia n. sp. F 2 0 0 2 0 1 2 1 1 0 1 0 1 1 1 0 0 - 0 0 1 1 1 1 1 1 0 1 2 0 0 2 1 1 2 3

215
Continuação apêndice III. Matriz de dados provenientes dos caracteres morfológicos levantados a
partir do exame de machos de Oxysarcodexia Townsend, 1917 (Diptera: Sarcophagidae) ( _ = não se
aplica; ? = “missing data”).
37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60 61 62 63 64 65 66 67 68 69 70 71 72 73
D. transita 1 1 0 0 1 1 - 0 1 1 1 1 1 0 0 1 1 1 - 1 - 1 0 1 0 0 1 - 1 1 1 - - - - - 0
R. belforti 1 1 1 2 1 1 - 1 1 1 1 1 1 1 - 1 1 1 - 1 - 1 0 0 0 0 1 - 1 2 1 - - - - - 0
O. admixta 1 1 1 1 1 1 0 1 1 1 0 0 1 1 - 1 1 1 - 0 1 1 0 1 0 1 0 0 0 2 0 0 1 0 1 2 1
O. adunca 1 0 1 0 1 1 1 1 1 1 1 0 1 1 - 1 1 1 - 0 0 0 1 0 0 0 1 - 1 0 0 0 2 0 0 2 0
O. afficta 1 0 1 1 1 1 1 1 1 1 0 0 1 0 0 1 1 1 - 0 1 1 1 1 0 0 1 - 0 2 0 0 2 1 - - 1
O. amorosa 1 1 1 1 1 1 1 0 1 1 1 0 1 1 - 1 1 1 - 0 1 1 0 0 0 0 0 0 0 2 0 1 2 0 1 0 0
O. angrensis 0 0 0 3 1 1 1 0 1 1 0 1 1 1 - 1 1 0 0 0 0 1 1 1 0 0 1 - 0 2 0 1 0 0 0 1 0
O. augusta 0 1 1 1 1 1 1 0 0 0 1 1 1 1 - 1 0 1 - 0 1 1 0 0 0 0 0 1 0 2 0 0 2 0 1 1 0
O. aura 0 0 1 2 1 1 0 0 1 1 1 0 1 1 - 1 1 1 - 0 1 2 0 1 0 0 1 - 1 2 1 - - 1 - - 1
O. aurata 0 0 1 1 0 1 1 1 1 1 1 0 1 0 2 0 1 1 - 0 1 2 1 1 0 0 1 - 0 1 0 0 2 0 0 0 0
O. avuncula 1 0 1 0 1 1 1 0 1 1 1 0 1 1 - 1 1 1 - 0 0 1 1 1 0 0 1 - 1 1 0 1 1 0 0 1 0
O. bakeri 1 0 1 1 1 1 0 1 0 1 0 0 0 1 - 1 1 1 - 1 - 0 1 1 0 0 1 - 0 2 0 1 1 0 1 1 1
O. berlai 1 1 1 1 1 1 1 0 0 1 1 1 1 1 - 1 1 1 - 0 1 0 0 1 0 0 1 - 0 2 0 1 2 0 1 2 0
O. bicolor 1 1 0 3 1 0 0 1 1 1 1 1 1 0 2 1 0 0 0 0 1 1 0 0 0 1 1 - 1 0 0 0 0 0 1 0 0
O. carvalhoi 1 1 1 0 1 1 1 1 1 0 0 0 0 1 - 1 1 1 - 0 0 1 0 1 0 1 0 0 0 2 0 0 2 0 1 0 0
O. chaetopygialis 0 0 0 3 1 1 1 1 1 1 2 1 1 1 - 1 1 0 1 0 0 2 0 1 0 1 1 - 1 0 1 - - 0 1 1 1
O. cingarus 1 1 0 3 1 0 0 0 1 1 2 0 1 1 - 1 1 1 - 0 0 1 0 0 0 1 1 - 0 2 1 - - 0 0 0 1
O. conclausa 0 0 0 0 1 1 0 1 0 1 1 0 1 0 1 1 1 1 - 0 1 0 1 0 0 0 1 - 2 2 0 1 1 0 0 1 1
O. confusa 1 0 0 0 1 0 0 0 1 1 1 0 1 1 - 1 1 1 - 0 0 1 0 1 0 1 1 - 1 1 0 0 0 0 1 1 0
O. culmiforceps 0 0 1 1 1 0 1 0 0 0 0 1 1 1 - 1 0 1 - 0 0 1 1 0 0 1 1 - 0 1 0 0 1 0 0 0 0
O. culminata 1 0 0 0 0 0 0 0 0 1 0 1 1 1 - 1 1 1 - 0 0 1 1 1 0 1 1 - 1 1 0 0 2 0 0 1 1
O. diana 1 1 0 0 1 1 1 0 1 1 1 1 1 1 - 1 1 1 - 0 0 1 1 0 0 0 1 - 1 0 0 0 2 0 1 0 1
O. fluminensis 1 1 1 0 1 1 1 0 0 0 1 1 1 1 - 1 1 1 - 0 1 1 1 0 0 0 0 1 0 2 0 1 1 0 1 1 0
O. fringidea 0 0 0 0 1 1 1 0 1 1 0 0 1 1 - 1 1 1 - 0 1 1 1 1 1 0 1 - 1 1 0 1 1 0 0 1 0
O. galeata 1 0 0 3 1 0 0 1 1 1 0 0 1 0 2 1 0 1 - 0 1 1 1 0 0 1 1 - 0 2 0 0 1 0 1 1 1
O. grandis 1 0 0 0 1 1 1 0 1 0 1 1 1 1 - 1 1 0 0 0 0 0 0 1 0 0 0 1 0 2 0 1 2 0 1 1 0
O. injuncta 1 1 1 2 1 1 1 0 0 0 1 0 1 1 - 1 0 0 0 0 1 0 0 1 0 0 0 1 0 2 0 2 0 0 1 0 0
O. insolita 1 2 0 0 1 0 1 1 0 1 1 0 1 1 - 1 1 0 0 0 1 1 0 1 0 0 1 - 0 2 0 1 2 0 1 0 0
O. intona 0 0 1 2 0 1 1 1 1 1 1 1 0 0 2 0 1 1 - 0 1 0 1 0 0 1 1 - 0 1 0 0 0 0 0 0 1
O. major 1 0 0 0 1 0 1 1 0 1 1 0 1 1 - 1 1 0 0 0 1 0 1 0 0 0 1 - 0 2 0 1 2 0 0 0 1
O. mitifica 1 0 0 3 1 0 0 1 1 0 1 1 1 1 - 1 1 1 - 0 0 1 1 0 0 0 1 - 0 1 0 1 0 0 0 1 1
O. modesta 1 1 1 0 1 1 0 0 0 1 1 1 0 1 - 1 1 1 - 0 0 0 0 1 0 0 1 - 0 1 0 0 0 0 1 2 0
O. nitida 0 1 1 1 1 1 0 1 1 1 0 0 1 1 - 0 0 1 - 0 0 2 1 1 0 0 1 - 2 2 1 - - 1 - - 1
O. notata 1 1 1 0 1 1 0 1 0 1 2 1 0 1 - 0 0 1 - 0 1 1 0 1 0 0 1 - 0 2 0 0 2 0 1 2 1
O. occulta 1 0 1 0 1 1 1 1 1 1 1 1 1 1 - 1 1 0 0 0 0 0 1 0 0 0 1 - 0 1 1 - - 0 0 2 1
O. parva 1 1 0 3 1 0 0 0 1 1 1 1 1 1 - 1 0 1 - 0 0 1 1 1 0 1 1 - 1 0 1 - - 0 0 0 1
O. paulistanensis 1 1 1 1 1 1 1 0 0 1 1 1 1 0 0 1 1 1 - 0 1 1 0 1 0 0 0 0 0 2 0 2 2 0 1 1 0
O. peltata 1 1 1 0 0 1 1 1 1 1 0 0 1 0 2 0 1 1 - 0 1 0 1 1 0 1 1 - 0 1 0 0 0 0 0 1 1
O. perneta 1 1 0 3 1 0 1 0 1 1 1 0 1 0 0 1 1 1 - 0 0 1 0 1 0 0 1 - 1 0 0 0 0 0 1 0 0
O. plebeja 1 1 0 0 1 1 0 0 0 1 1 0 1 1 - 1 1 1 - 0 0 1 0 1 0 1 1 - 0 2 0 0 2 0 1 1 1
O. riograndensis 1 1 1 0 1 1 0 0 0 1 2 1 1 0 1 1 1 1 - 0 0 1 0 1 0 0 0 0 1 2 0 1 2 0 1 0 0
O. sarcinata 1 2 1 0 1 0 1 0 1 1 1 1 1 1 - 1 1 1 - 0 0 0 0 1 1 0 1 - 0 2 0 1 2 0 0 1 1
O. similata 1 1 1 2 1 1 0 1 1 1 1 0 1 1 - 1 1 1 - 0 0 1 0 0 0 0 0 0 0 2 0 1 2 0 1 1 0
O. terminalis 0 1 1 0 1 1 1 1 1 1 2 1 1 1 - 1 1 1 - 0 0 2 0 1 0 0 0 0 0 1 0 0 1 1 - - -
O. thornax 1 0 1 1 1 1 1 1 0 1 1 0 1 0 1 1 1 1 - 0 1 0 1 0 0 0 1 - 2 2 0 0 1 0 0 1 0
O. timida 1 0 1 1 0 1 1 1 1 1 0 0 1 0 0 1 1 1 - 0 1 0 1 1 1 1 1 - 0 2 0 1 1 0 0 1 0
O. trivialis 1 0 0 0 1 1 1 0 1 1 1 0 1 1 - 1 1 1 - 0 1 0 1 1 0 0 1 - 0 1 0 0 2 0 1 2 1
O. varia 0 1 1 1 1 0 1 1 1 1 0 1 1 0 2 1 1 1 - 0 0 0 0 1 1 0 1 - 1 2 0 1 1 0 1 1 0
O. ventricosa 1 2 1 1 1 0 1 0 0 1 0 0 1 1 - 1 1 0 0 0 0 1 1 1 0 1 1 - 0 2 0 0 0 0 1 1 0
O. vittata 1 1 1 2 1 1 ? 1 1 1 2 1 0 0 1 0 0 1 - 0 0 2 1 0 0 0 1 - 2 2 1 - - 1 - - 1
O. xanthosoma 1 1 1 1 1 1 1 0 1 1 1 1 1 1 - 1 1 1 - 0 1 0 1 1 0 0 0 0 0 2 0 1 2 0 1 1 0
O. zayasi 1 2 0 3 1 0 0 0 1 1 2 0 1 1 - 1 1 1 - 0 0 1 1 0 0 1 1 - 0 1 0 0 2 0 1 2 1
Oxysarcodexia n. sp. A 1 1 1 1 1 1 0 1 1 1 1 1 1 1 - 1 1 0 0 0 1 1 0 1 0 0 0 0 0 2 0 1 2 0 1 1 1
Oxysarcodexia n. sp. B 1 1 1 1 1 1 0 0 0 1 1 1 1 1 - 1 1 0 0 0 0 1 1 1 0 0 1 - 0 2 0 0 2 0 1 1 1
Oxysarcodexia n. sp. C 1 1 1 2 1 1 0 0 1 1 1 1 0 1 - 1 1 0 0 0 0 0 0 1 0 0 1 - 0 2 0 0 2 0 1 1 -
Oxysarcodexia n. sp. F 1 1 1 1 1 1 0 1 0 1 0 0 1 1 - 1 1 0 0 0 1 1 0 1 0 1 1 - 0 2 0 0 2 0 1 1 1

216

217
7. CONSIDERAÇÕES FINAIS
O conspecto apresentado para Oxysarcodexia, visou contribuir para uma melhor
compreensão da variedade morfológica dos machos deste gênero, assim como fornecer uma
atualização acerca da nomenclatura válida e sinonimização, distribuição geográfica e aspectos
biológicos das espécies pertencentes a esse gênero. Espera-se que o banco de dados pictórico
elaborado para os machos de Oxysarcodexia, associado às diagnoses, possa auxiliar no
processo de identificação de espécies, em especial daquelas morfologicamente próximas e
simpátricas, sendo um facilitador nos mais diversos estudos, os quais dependem da acurácia na
identificação, como, por exemplo, a entomologia forense.
A abordagem filogenética proposta representa um passo inicial para a compreensão das
relações dentro desse gênero, apontando paralelos nas variações morfológicas encontradas
entre as diferentes espécies, sugerindo agrupamentos interespecíficos, apesar do alto grau de
homoplasia presente nas espécies. Além disso, a assimetria na genitália dos machos revelou-se
uma característica independente que surgiu várias vezes durante a diversificação das espécies.
Vale ressaltar que o estudo morfológico das fêmeas de Oxysarcodexia à luz da
filogenia, assim como das larvas, poderá contribuir significativamente para um melhor
entendimento da relação entre as espécies. Da mesma forma, a análise morfológica de fêmeas
e a observação do comportamento de cópula de casais de espécies cujos machos apresentam a
assimetria na genitália possibilitará esclarecer como a assimetria evolui dentro desse gênero.

218

219
8. REFERÊNCIAS BIBLIOGRÁFICAS
Amat, E. 2010. Notes on necrophagous flies (Diptera: Calyptratae) associated to fish carrion in
Colombian Amazon. Acta Amazonica, 40 (2): 397–400.
Amorim, D.S. 2002. Fundamentos de Sistemática Filogenética. Holos Editora, Ribeirão Preto, São
Paulo, Brasil. 154pp.
Amorim, D.S. 2009. Neotropical Diptera diversity: richness, patterns, and perspectives. In: Pape, T.,
Bickel, D. & Meier, R. 2009. Diptera Diversity: status, challenges and tools. Brill, Portland, 71–
97.
Amorim, D.S. 2012. Biogeografia da Região Neotropical. In: Rafael, J.A., Melo, G.A.R., Carvalho,
C.J.B., Casari, S. & Constantino, C. (eds.), Insetos do Brasil, diversidade e taxonomia. Holos
Editora, Ribeirão Preto, São Paulo, Brasil, pp. 112–132.
Battán-Horenstein, M., Linhares, A.X., Ferradas, B.R. & Garcia, D. 2010. Decomposition and dipteran
succession in pig carrion in central Argentina: ecological aspects and their importance in forensic
science. Medical and Veterinary Entomology, 24: 16–25.
Battán-Horenstein, M., Rosso, B. & García, M.D. 2012. Seasonal structure and dynamics of
sarcosaprophagous fauna on pig carrion in a rural area of Cordoba (Argentina): their importance in
forensic science. Forensic Science International, 217: 146–156.
Beuter, L., Fernandes, P.A., Barros, P.B., Souza, C.R. & Mendes, J. 2012. Insetos de potencial
importância forense e na saúde pública em região urbana de Minas Gerais: frequência relativa e
variação sazonal de fauna atraída e criada em carcaças de roedores. Revista de Patologia Tropical,
41 (4): 480–490.
Brown, B. V. 2009. Introduction. In: Brown, B.V., Borkent, A., Cumming, J.M., Wood, D.M.,
Woodley, N.E. & Zumbado, M.A. (eds.), Manual of American Central Diptera. Vol. 1. NCR
Research Press, Ottawa, Ontario, Canada, pp. 1–7.
Campobasso, C.P.; Di Vella, G. & Introna, F. 2001. Factors affecting decomposition and Diptera
colonization. Forensic Science International, 120: 18–27.
Carrera, M. 1944. Relação de alguns dípteros capturados em Monte Alegre, Estado de São Paulo.
Papéis avulsos do Departamento de Zoologia, 6 (5): 37–50.
Carvalho, C.J.B., Rafael, J.A., Couri, M.S. & Silva, V.C. 2012. Diptera. In: Rafael, J.A., Melo, G.A.R.,
Carvalho, C.J.B., Casari, S. & Constantino, C. (eds.), Insetos do Brasil, diversidade e taxonomia.
Holos Editora, Ribeirão Preto, São Paulo, Brasil, pp. 701–743.
Carvalho, L.M.L. & Linhares, A.X. 2001. Seasonality of insect succession and pig carcass
decomposition in a natural forest area in southeastern Brazil. Journal of Forensic Sciences, 46 (3):
604–608.

220
Carvalho, L.M.L., Thyssen, P.J., Goff, M.L. & Linhares, A.X. 2004. Observations on the succession
patterns of necrophagous insects onto a pig carcass in an urban area of Southeastern Brazil.
Aggrawal’s Internet Journal of Forensic Medicine and Toxicology, 5 (1): 33–39.
Carvalho, L.M.L., Thyssen, P.J., Linhares, A.X. & Palhares, F.B. 2000. A checklist of arthropods
associated with carrion and human corpses in southeastern Brazil. Memórias do Instituto Oswaldo
Cruz, 95 (1): 135–138.
Çiftçioğlu, N.; Altintaș, K. & Haberal M. 1997. A case of human orotracheal myiasis caused by
Wohlfahrtia magnifica. Parasitology Research, 83: 34–36.
Constamagna, R., Visciarelli, E.C, Lucchi, L.D., Basabe, N.E., Esteban, M.P. & Oliva, A. 2007.
Aportes al conocimiento de los dípteros ciclorrafos em el área urbana de Bahía Blanca (província
de Buenos Aires), Argentina. Revista del Museo Argentino de Ciencias Naturales, 9 (1): 1–4.
Cornaby, B.W. 1974. Carrion reduction by animals in contrasting tropical habitats. Biotropica, 6 (1),
51–63.
Couri, M.S., Lamas, C.J.E., Aires, C.C.C., Mello-Patiu, C.A., Pamplona, D.M. & Magno, P.D. 2000.
Diptera from Serra do Navio (Amapá, Brasil): Asilidae, Bombyliidae, Calliphoridae, Micropezidae,
Muscidae, Sarcophagidae, Stratiomyiidae, Syrphidae, Tabanidae and Tachinidae. Revista
Brasileira de Zoociências, 2 (1): 91–100.
Cruz, T. M. 2008. Diversidade e sucessão ecológica de insetos associados à decomposição animal em
um fragmento de Mata Atlântica de Pernambuco. Dissertação de Mestrado. 102 fls. Universidade
Federal de Pernambuco, Recife, Pernambuco, Brasil.
Dias, E.S., Neves, D.P. & Lopes, H.S. 1984. Estudos sobre a fauna de Sarcophagidae (Diptera) de Belo
Horizonte – Minas Gerais I- Levantamento Taxonômico e Sinantrópico. Memórias do Instituto
Oswaldo Cruz, 79 (1): 83–91.
Dutto, M. & Bertero, M. 2010. Traumatic myiasis from Sarcophaga (Bercaea) cruentata Meigen, 1826
(Diptera, Sarcophagidae) in a hospital environment: reporting of a clinical case following
polytrauma. Journal of Preventive Medicine and Hygiene, 51 (1): 50–52.
Evenhuis, N.L., Pape, T., Pont, A.C. & Thompson, F.C. (eds.) 2007. Biosystematic Database of World
Diptera, Version 10. Available at http://www.diptera.org/biosys.htm. apud Pape, T., Bickel, D. &
Meier, R. (2009) Diptera Diversity: status, challenges and tools. Brill, Portland, 459pp.
Evenhuis, N.L., Pape, T., Pont, A.C. & Thompson, F.C. (eds.) 2007. Biosystematic Database of World
Diptera, Version 10. Available at http://www.diptera.org/biosys.htm. apud Amorim, D.S. 2009.
Neotropical Diptera diversity: richness, patterns, and perspectives. In: Pape, T., Bickel, D. &
Meier, R. 2009. Diptera Diversity: status, challenges and tools. Brill, Portland, 71–97.
Ferreira, M.J.M. 1978. Sinantropia de dípteros muscóideos de Curitiba, Paraná. I. Calliphoridae.
Revista Brasileira de Biologia, 38: 445–454.

221
Figueiredo, R.R.; Dorf, S.; Couri, M.S.; Azevedo, A.A. & Mossumez, F. 2002. Corpos estranhos
animados em otorrinolaringologia. Revista Brasileira de Otorrinolaringologia, 68 (5): 722–728.
Giroux, M., Pape, T. & Wheeler, T.A. 2010. Towards a phylogeny of the flesh flies (Diptera:
Sarcophagidae): morphology and phylogenetic implications of the acrophallus in the subfamily
Sarcophaginae. Zoological Journal of the Linnean Society, 158: 740–778.
Greenberg, B. 1971. Flies and diseases. Ecology, classification and biotic association. Vol. 1.
Princeton: Princeton University, 856p.
Greenberg, B. 1973, Flies and diseases. Biology and disease transmission. Vol. 2. Princeton, Princeton
University, 447p.
Guimarães, J.H. & Papavero, N. 1999. Myiasis in man and animals in the Neotropical region:
bibiographic database. São Paulo: Editora Plêaide/FAPESP, 1 ed., 308pp.
Leão, R.N.Q; Fraiha, H.; Cruz, J.P.N. & Tibana, R. 1996. Miíase uretral por Sarcodexia lambens
(Wiedemann, 1830) (Diptera: Sarcophagidae): relato de um caso amazônico. Revista Paraense de
Medicina, 10 (1): 27–29.
Linhares, A.X. & Thyssen, P.J. 2007. Miíases de importância médica: moscas e entomologia forense.
In: De Carli, G.A. (org.) Parasitologia Clínica: seleção de métodos e técnicas de laboratório para o
diagnóstico das parasitoses humanas. 2ª ed., São Paulo: Atheneu, 709–730.
Liu, D. & Greenberg, B. 1989. Immature stages of some flies of forensic importance. Annals of the
Entomological Society of America, 82 (1): 80–93.
Lopes, H.S. 1969. A Catalogue of the Diptera of the Americas South of the United States: Family
Sarcophagidae. Departamento de Zoologia, Secretaria da Agricultura, 103, 88p.
Lopes, H.S. 1982. The importance of the mandible and clypeal arch of the first instar larvae in the
classification of the Sarcophagidae (Diptera). Revista Brasileira de Entomologia, 26 (3/4): 293–
326.
Mégnin, J. La faune des cadavres: application de l'entomologie a la medecine legale. Encyclopedie
Scientifique des Aides Memoires. Masson et Gauthiers-Villars, Paris, 1894. 214p.
Monteiro-Filho, E.L.A. & Pernereiro, J.L. 1987. Estudo de decomposição e sucessão sobre uma
carcaça animal numa área do estado de São Paulo, Brasil. Revista Brasileira de Biologia, 47 (3):
289–295.
Nuorteva, P. 1977. Sarcosaprophagous insects as forensic indicators. In: Forensic medicine: a study in
trauma and environmental hazards. vol. II. W.B. Saunders Company, 1072–1095.
Pamplona, D., Maia, V.C., Couri, M.S., Lamas, C.J.E. & Aires, C.C.C. 2000. A survey of díptera on
Paquetá Island, Rio de Janeiro, Brazil. Entomologist`s Monthly Magazine, 136: 169–175.

222
Pape, T. & Dahlem, G.A. 2010. Sarcophagidae. In: Brown, B.V., Borkent, A., Cumming, J.M., Wood,
D.M., Woodley, N.E. & Zumbado, M. (eds), A Manual of Central American Diptera. Vol. 2. NRC
Research Press, Ottawa, pp. 1313–1335.
Pape, T. 1996. Catalogue of the Sarcophagidae of the world (Insecta: Diptera). Memoirs on
Entomology, International, 8: 1–558.
Pérez, S.P., Duque, P., Wolff, M. 2005. Successional and behavior and occurrence matrix of carrion-
associated arthropods in the urban área of Medellín, Colombia. Journal of Forensic Science, 50 (2):
448–453.
Rafael, J.A.; Aguiar, A.P. & Amorim, D.S. 2009. Knowledge of insect diversity in Brazil: challenges
and advances. Neotropical Entomology, 38 (5) : 565–570.
Ramírez-Mora, M.A., Buenaventura, E., Gómez-P, L.M. & Amat, E. (2012) Uptadet cheklist and new
records of Calyptrate carrion flies (Diptera, Schizophora) from Valle de Aburrá and other localities
in Colombia. Entomotropica, 27 (1), 27–35.
Roback, S.S. 1954. The evolution and taxonomy of the Sarcophaginae (Diptera, Sarcophagidae).
Illinois Biological Monographs, 23: 1–181.
Shewell, G.E. 1987. Sarcophagidae. In: McAlpine, J.F., Peterson, B.V., Shewell, G.E., Teskey, H.J.,
Vockeroth, J.R. & Wood, D.M. (eds.), Manual of Nearctic Diptera. Vol. 2. Monograph 27,
Agriculture Canada, pp. 1159–1186.
Smith, K.G.V. 1986. A manual of Forensic Entomology. Cornell University Press, Ithaca, NY, 205 pp.
Sousa, J.R.P., Esposito, M.C. & Carvalho-Filho, F.S. 2011. Composition, abundance and richness of
Sarcophagidae (Diptera: Oestroidea) in forests and forest gaps with different vegetation cover.
Neotropical Entomology, 40 (1): 20–27.
Souza, A.M. & Linhares, A.X. 1997. Diptera and Coleoptera of potential forensic importance in
Southeastern Brazil: relative abundance and sazonality. Medical and Veterinary Entomology, 11:
8–12.
Thyssen, P.J.; Moretti, T.C.; Ueta, M.T. & Ribeiro, O.B. 2004. O papel de insetos (Blattodea, Diptera e
Hymenoptera) como possíveis vetores mecânicos de helmintos em ambiente domiciliar e
peridomiciliar. Cadernos de Saúde Pública, 20: 1096–1102.
Turhan, V.; Ulaş, U.H.; Aydin, A.F.; Eyigün, C.P.; Araz, E.; Tanyüksel, M. & Pahsa, A. 2007. An
intestinal myiasis case of flesh fly (Sarcophaga haemorrhoidalis). The Anatolian Journal of
Clinical Investigation, 1 (4): 270–272.
Tuygun, N.; Taylan-Özkan, A.; Tanir, G. & Mumcuoğlu, K.Y. 2009. Furuncular myiasis in a child
caused by Wohlfahrtia magnifica (Diptera: Sarcophagidae) associated with eosinophilia. The
Turkish Journal of Pediatrics, 51: 279–281.

223
Verves, Y. 1989. Prof. Hugo de Souza Lopes and the modern system of Sarcophagidae (Diptera).
Memórias do Instituto Oswaldo Cruz, 84: 529–545.
Yazgi, H.; Uyanik, M.H.; Yoruk, O. & Aslan, I. 2009. Aural myiasis by Wohlfahrtia magnifica: case
report. The Eurasian Journal of Medicine, 41: 194–196.
Zhang, Z.-Q. 2013. Phylum Arthropoda. In: Zhang, Z.-Q. (Ed.) Animal Biodiversity: An outline of
higher-level classification and survey of taxonomic richness (Addenda 2013). Zootaxa, 3703, 1–82.
Zumpt, F. Myiasis in man and animals in the Old World. London: Butterworths, 1965. 267pp.